An introduction to needlestick protection and safety syringes
13 June 2018
The need
Needlestick injuries are second only to back injuries as a cause of injury to healthcare workers (HCW), with up to 1,000,000 cases estimated annually in the EU [1]. The risk transmission of blood-borne viruses has been shown to be around 1 in 3 if the patient is Hepatitis B Virus positive, 1 in 30 if Hepatitis C Virus positive and 1 in 300 if HIV positive [2, 3]. Even when infections are not transmitted such an injury can cause considerable fear and stress [4].
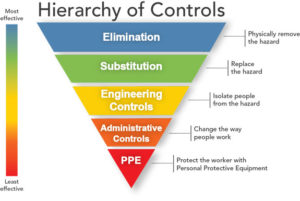
In many cases, it is not feasible to eliminate injections or use another administration method as a substitute. Therefore, the hazard is most effectively handled by engineering solutions which separate those at risk from the sharps (Figure 1). Research by the UK’s Health and Safety Executive has found that education and training related to safer sharps is only effective when combined with safer sharps devices [5] and the UK’s Health Protection Agency recommends that Primary Care Trusts and hospitals adopt safety devices in place of conventional devices [2]. In the US, the CDC estimated that up to 88% of sharps injuries in hospitals could be prevented by using safer medical devices [6].
Figure 1 – Hierarchy of hazard controls with increasing effectiveness (Source: National Institute for Occupational Safety and Health)
The legal requirements
Many regulators around the world require needlestick protection, and others are in the process of implementing it. Examples are:
- US Needlestick Safety and Prevention Act (2000)
- EU Directive 2010/32/EU – prevention from sharp injuries in the hospital and healthcare sector
The international standard ISO 23908:2011 defines the requirements and test methods for sharps protection features on single-use hypodermic needles, introducers for catheters, and needles used for blood sampling.
‘Active’ and ‘passive’ solutions
Broadly, solutions can be classed as:
- Active (the user must take some type of action to initiate the feature) or
- Passive (the feature is activated automatically, without user intervention).
Active solutions rely on the HCW completing an extra step, which relies on training and changing existing behaviours. The UK National Health Service Employers organisation recommends that needle safety mechanisms should be an integral part of the device, require little change of technique and are preferably activated automatically or with a single hand [7].
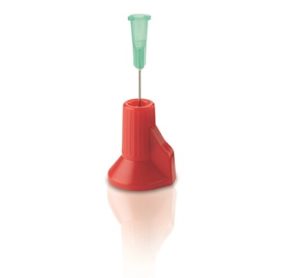
A simple active solution is to cap the exposed needle with a shield after use, such as Smith Medical’s Point-Lok® needle protection device (Figure 2). The device sits on a flat surface to accept and lock onto the exposed needle.
Figure 2 – Smith Medical’s Point-Lok® needle protection device
Active Sharps Injury Protection (sheath)
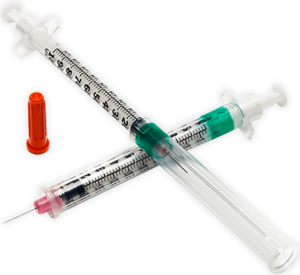
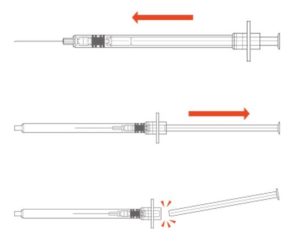
Syringes with integral sharps injury protection (SIP) often use a cover that shields the user from the needle after use. This cover can be an extendable tube (Figure 3). The syringe is pulled back into the sheath, which clicks and locks into place. The sheath covers the outer surface of the barrel during the injection. These syringes should be held by the flange, so the sheath is not displaced before the end of the injection. This may require a change in habit for HCWs. Example of this type of safety syringe include:
- BD Safety-Lok™ Syringe (1, 3, 5 & 10 mL, takes luer Lok needles)
- EasyTouch® ShealthLock™ Safety Syringe (fixed needle 1 & 3 mL, exchangeable needle 3, 5 & 10 mL)
- Medtronic Monoject™ Safety Syringe (with needle, for insulin and tuberculin)
- Sol-Millenium Sol-Guard™ Safety Syringe (with needle, for insulin and tuberculin)
- UltiMed Ulticare® Safety Syringe (1, 1.5 & 3 mL permanently attached needle)
- Vogt Medical VM® Safety syringe (1, 3, 5 & 10 mL)
Figure 3 – BD Safety-Lok™ Syringe, an example of a safety syringe with a tubular SIP feature
A similar solution is the Raumedic RauSafe, which uses a telescopic sheath that is manually extended and locked out over the needle after the injection. This avoids covering the external surface of the barrel before and during the injection
Figure 4 – Raumedic RauSafe shown with the protective sheath extended
Active Sharps Injury Prevention (hinge)
The needle safety feature can also take the form of a hinged cover. The cover can be pushed down with a finger or against a hard surface so only one hand is required. The cover can be incorporated into the syringe, such as:
- EasyTouch® FlipLock™ Safety Syringe (fixed needle, 1 & 3 mL, exchangeable needle 3, 5 & 10 mL)
- Medtronic Magellan™ Safety Syringe (fixed needle insulin & turerculin)
- Vogt Medical VM® Safety syringe (1, 3, 5, 10 & 20 mL)
The same feature can be incorporated into attachable needles, such as:
- BD Eclipse™ Safety Needle (18 – 30G)
- EasyTouch® FlipLock™ Safety Syringe (18 – 31G)
- Medtronic Monoject™ Safety Needle (18 – 30G)
- Terumo SurGuard®3 Safety Needle (18 – 30G)
- Smiths Medical Jelco® Needle-Pro® EDGE™ Safety Needle (18 – 30G)
- Sol-Millenium Sol-Care™ Safety Needle (18 – 30G)
- Vogt Medical VM® Safety needle (20 – 27G)
A potentially cost-effective and versatile solution is Schreiner MediPharm’s Needle-Trap, which incorporates the hinged cover into the syringe label.
Figure 5 – Hinged SIP covers (left: EasyTouch® FlipLock™ Safety Syringe, middle: Terumo SurGuard®3 Safety Needle, right: Schreiner MediPharm Needle-Trap)
There are some multi-hinged needle covers available that can facilitate easier one-handed application:
- BD SafetyGlide™ Needle
- Medtronic Magellan™ Safety Needle
Figure 6 – Left: BD SafetyGlide™ Needle, Right: Medtronic Magellan™ Safety Needle
Active Sharps Injury Prevention (needle retraction)
Instead of shielding the needle after use, the needle can be retracted into the syringe after the injection. There are products available to do this manually, by pulling back on the plunger rod, such as:
- EasyTouch® Retractable Safety Syringe
- Numedico ClickZip™ Needle Retractable Safety Syringe
- Sol-Millennium SOL-CARE™ Safety Syringe
Figure 7 – EasyTouch® Retractable Safety Syringe instructions (adapted from http://mhcmed.com/products/easytouch-safety-products/safety-syringes/)
Similar retraction technology is available as a needle with luer lock connection, such as in the Retractable technologies EasyPoint® Needle. The sharp is automatically captured in an adjacent chamber but an additional user step is required to activate this process.
Figure 8 – EasyPoint® retractable needle
Passive Sharps Injury Protection (needle retraction)
There are syringes available which retract the needle into the barrel automatically using a spring, eliminating any extra user steps. Examples include:
- BD Intergra™ Syringe (3 mL, requires detachable BD Integra needles)
- Retractable technologies VanishPoint® Syringe (attached needle, 0.5, 1, 3, 5, and 10 mL)
- DMC Medical SureSafe™ Syringe (fixed needle 0.5 & 1 mL, changeable needle 3ml, 5ml & 10ml)
Figure 9 – BD Intergra™ Syringe retracting syringe
Passive Needle Safety devices
An alternative solution is to encompass the syringe in a safety device.
The BD Preventis™ allows a 0.5 ml or 1.0 ml pre-filled syringe to be packaged inside a casework that incorporates an automatic safety lock system. The safety device uses a spring to extend a protective sheath after injection.
Figure 10 – BD Preventis™ needle shielding system
Such single-use devices can be used with syringes and shift the act of shielding the needle from the user to the device. Typically, a syringe is inserted inside such a device and the injection is performed by depressing a plunger rod, then after the injection is complete a spring acts to withdraw the syringe and shield the needle.
In the Biocorp Newguard, the user’s push on the plunger rod acts on a spring which causes a needle retraction and lockout after injection. The Nemera Safe’n’Sound and the BD Ultrasafe™ does not require the user to work against a spring but uses an unclipping mechanism release the compressed spring at end of injection to activate the retraction.
The Owen Mumford Unisafe™ avoids using a spring altogether and retracts the syringe by transferring the plunger rod stroke through a threaded interface. This can help the user see the syringe and its contents before and during the injection.
Figure 11 – Left: Nemera Safe’n’Sound, Middle: Biocorp New guard, Right: Owen Mumford Unisafe™
If you would like to know more about needle safety devices, or have a need to procure or develop one, please get in touch. Springboard develops injection technologies, and conducts technology scouting, technology procurement, due diligence and usability engineering projects for our clients.
References
[1] European Parliament. Preventing needle-stick injuries in the health sector, 11th February 2010.
[2] Health Protection Agency, Eye of the Needle – United Kingdom Surveillance of Significant Occupational Exposures to Bloodborne Viruses in Healthcare Workers,
November 2008
[3] UK Health Departments, Guidance for Clinical Health Workers: Protection Against Infection with Blood-borne Viruses, April 1998
[4] Royal College of Nursing, Sharps safety – RCN Guidance to support the implementation of The Health and Safety (Sharp Instruments in Healthcare
Regulations), 2013
[5] Health and Safety Executive, An evaluation of the efficacy of safer sharps devices, 2012
[6] U.S. Occupational Safety and Health Administration, Needlestick/Sharps Injuries, Accessed March 2018
[7] NHS Employers, Managing the risks of sharps injuries, December 2015