Strategic insights into the device market
Client: Novartis
Description: Novartis needed a comprehensive view of the device options available to them that could deliver their therapies.
Activities: Extensive knowledge of the drug delivery device landscape so that valuable insights could be delivered to Novartis quickly. Specific experience in developing novel drug delivery devices so we could identify the benefits and risks of the various options. Independence from manufacturers, giving an impartial viewpoint.
The problem
Cell and gene therapies typically cannot be delivered through the GI tract because either:
- The biological activity of the therapy would be reduced or eliminated by the action of the GI tract, and/or
- The cell or gene therapy needs to be delivered to a specific part of the body, for example directly into a malignant tumour.
Novartis has a valuable and rapidly growing portfolio of cell and gene therapies. However, they could not be sure that they a comprehensive view of the device options available to them. They needed to know which device platforms, if any, could deliver the therapies in development, and if they could leverage any current devices.

Results
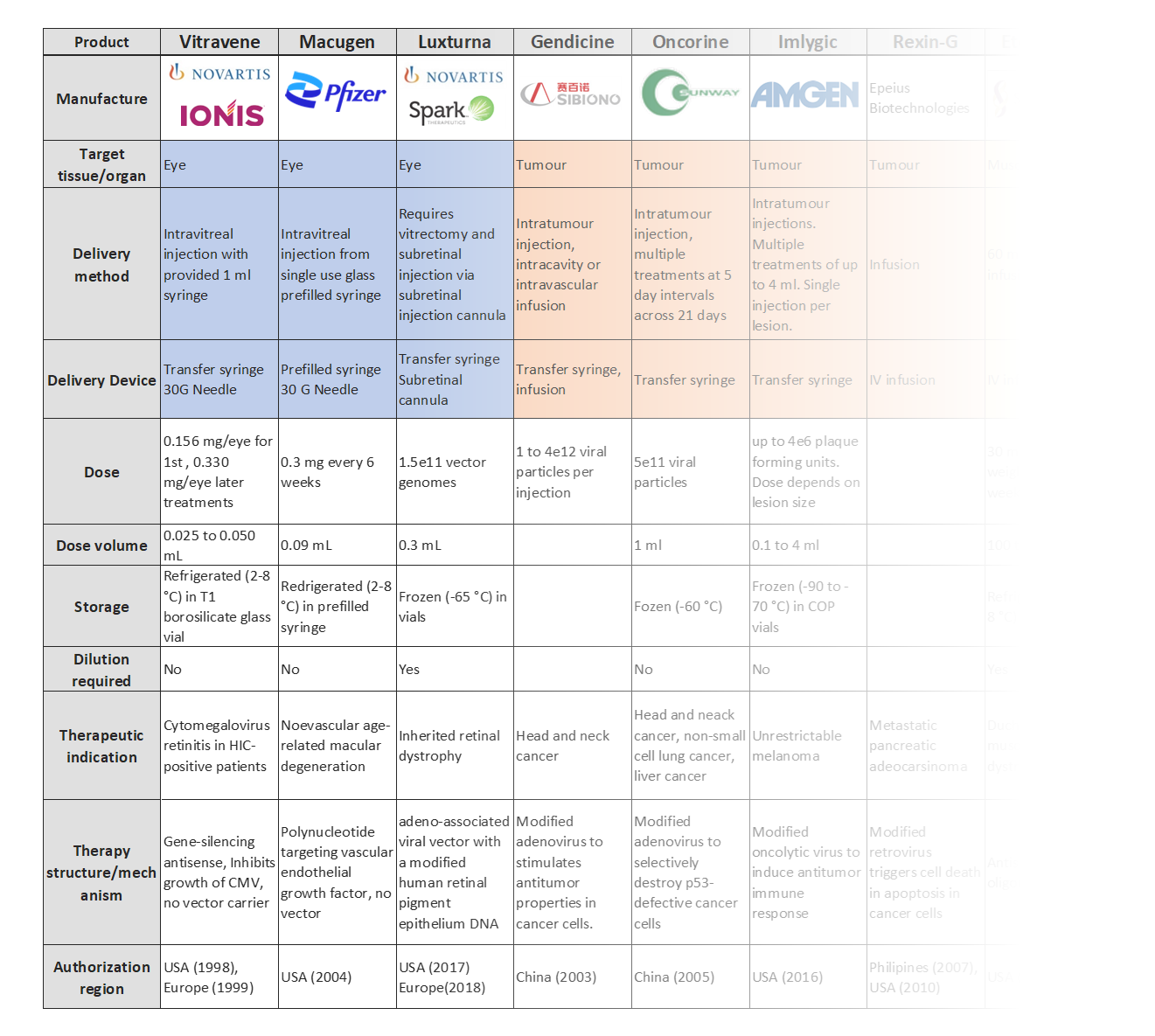
For each gene therapy within scope of the project, Springboard created a report and database covering:
- A table of devices that are either already available or are at an early research phase.
- A description of each device that included:
- Site and method of injection.
- Manufacturer or academic group.
- Characteristics e.g., dead volume, materials, deliverable volume.
- Technology readiness level using Novartis’ scale.
- Known advantages and disadvantages.
- An opinion of the preferred device and recommendations about further development of the preferred device.