Who ate all the (Christmas mince) pies?
3 December 2024
The holiday season is upon us, and (over) indulgence is in full swing—our team’s chocolate and mince pie consumption has…

Are you going to Med Tech Ireland, CPHI, PDA and PODD?
11 September 2024
The conference season is well underway and the Springboard and Sanner team are exhibiting at a few conferences throughout the…

Closing the Loop: The Latest on Artificial Pancreases
20 July 2024
Closed-loop insulin delivery systems, also known as artificial pancreas systems, are currently in an exciting phase of technological innovation and…

Through Thick and Thin: Modelling the Delivery of Non-Newtonian Formulations
24 June 2024
The pharmaceutical industry is currently undergoing a period of rapid innovation, propelled by several factors. These include advancements in the…

Pharmaceutical Industry Sustainability Targets, One Year On
22 May 2024
In the October 2022 “Drug Delivery & Environmental Sustainability” issue of ONdrugDelivery, We discussed climate-related targets set by 10 major pharmaceutical companies, along…
Shaping the future of healthcare: Data, AI and Us
8 May 2024
Mike Beck, our Head of Electronics spent some time in Boston at the Device Talks Conference last week. Here are…
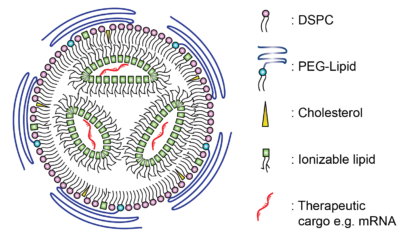
Bubble-Wrapped Remedies: How Lipid-Encased Therapeutics Could Transform Inhalation
30 April 2024
Introduction In a post-COVID-19 era we have witnessed a notable shift in vaccine development with the advent of lipid nanoparticle…

Springboard Pro to Host Networking Event for Startups in the Medical Device and Diagnostic Sphere
24 April 2024
Springboard Pro, a leading engineering consultancy, is pleased to announce its upcoming networking event tailored specifically for startups operating in…

Start up Grant Aims to Support Medical Device Industry Innovation
26 March 2024
Springboard Pro proudly announces the launch of its 2024 Start-up Grant. This program is specifically tailored to assist fledgling medical…