Shaping the future of healthcare: Data, AI and Us
8 May 2024
Mike Beck, our Head of Electronics spent some time in Boston at the Device Talks Conference last week. Here are…
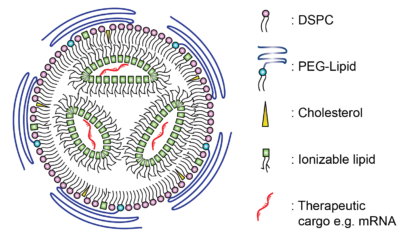
Bubble-Wrapped Remedies: How Lipid-Encased Therapeutics Could Transform Inhalation
30 April 2024
Introduction In a post-COVID-19 era we have witnessed a notable shift in vaccine development with the advent of lipid nanoparticle…

Springboard Pro to Host Networking Event for Startups in the Medical Device and Diagnostic Sphere
24 April 2024
Springboard Pro, a leading engineering consultancy, is pleased to announce its upcoming networking event tailored specifically for startups operating in…
Lab-On-Chip Devices: Materials Considerations
18 March 2024
Material selection and testing can often be one of the critical challenges to developing microfluidic devices for the healthcare and…

Bridging the gap: Adapting hospital equipment for home use
5 February 2024
The healthcare landscape is evolving, and one of the significant shifts is the increasing emphasis on home-based care. As technology…

The final frontier to improving low-pain injections
25 January 2024
Most people dislike injections. In some cases, that aversion comes from the pain experienced upon injection, and this discomfort can…

From Concept to Cure: Mastering Liquid Silicone Rubber for Medical Device Design
23 January 2024
Liquid silicone rubber (LSR) is frequently used in implants due to its bio-inert nature, meaning that it has a very…
How Energy Models trim 80% from Root Cause investigation time
12 December 2023
In the drug delivery sector, time really is money. Every day spent developing a drug delivery system increases development costs…

Can we trust AI in medical devices?
20 November 2023
Artificial intelligence (AI) and machine learning (ML) has the potential to revolutionise medical devices, and develop solutions that can scale…