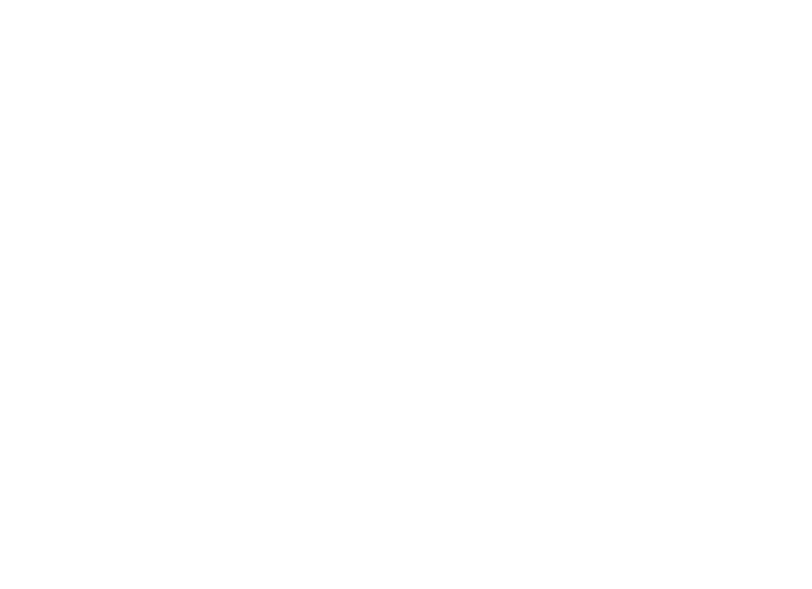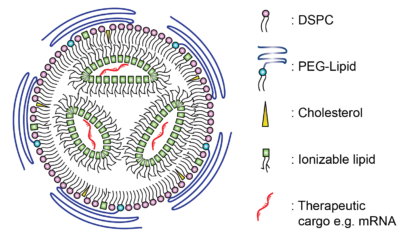
Bubble-Wrapped Remedies: How Lipid-Encased Therapeutics Could Transform Inhalation
30 April 2024
Introduction In a post-COVID-19 era we have witnessed a notable shift in vaccine development with the advent of lipid nanoparticle…

Springboard Pro to Host Networking Event for Startups in the Medical Device and Diagnostic Sphere
24 April 2024
Springboard Pro, a leading engineering consultancy, is pleased to announce its upcoming networking event tailored specifically for startups operating in…

Start up Grant Aims to Support Medical Device Industry Innovation
26 March 2024
Springboard Pro proudly announces the launch of its 2024 Start-up Grant. This program is specifically tailored to assist fledgling medical…

Superficial attraction: better membranes through surface science
6 March 2024
The importance of understanding surface properties Water is a multifaceted actor in our lives, being variously an ingredient in food,…

The final frontier to improving low-pain injections
25 January 2024
Most people dislike injections. In some cases, that aversion comes from the pain experienced upon injection, and this discomfort can…

Drug Delivery Trends for 2024
23 January 2024
Tom Oakley and Kamaal de Silva consider how keeping abreast of the latest trends can help the industry predict and prepare for…
How Energy Models trim 80% from Root Cause investigation time
12 December 2023
In the drug delivery sector, time really is money. Every day spent developing a drug delivery system increases development costs…

Human Factors Considerations For Your Emergency Use Drug Delivery Device
6 December 2023
Emergency use devices have been a long-standing part of the drug-device combination product market, with early autoinjectors being developed for…
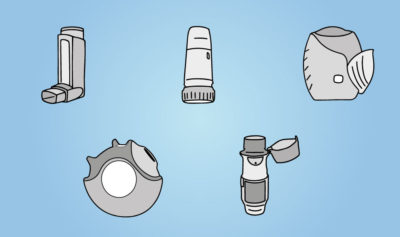
The end of the road for pMDIs?
22 November 2023
For decades the portable inhaler market has been dominated by two categories of inhaler: pressurised Metered-Dose inhalers (pMDIs) and Dry-Powder…