Nanopatterning for medical applications
6 September 2018
Nanotechnology appears in popular culture as a cure for everything from cancer to balding. In science nanotechnology is an umbrella term for a variety of structures and molecules used in optics, MEMS, materials, chemicals and some biological systems.
In this new blog series, we shall explore nanopatterning (the engineering of nanoscale structures on surfaces), its prevalence in nature, manufacture and application to medical devices.
Drawing inspiration from nature
 Figure 1 – Sunset moth scales macro by Johan J.Ingles-Le Nobel, Cropped, CC BY-NC-ND 2.0
Figure 1 – Sunset moth scales macro by Johan J.Ingles-Le Nobel, Cropped, CC BY-NC-ND 2.0
Nanoscale structure plays a fundamental role in numerous biological systems, and in some cases has developed to aid an organism’s survival and proliferation. Nanostructures can impact on the wetting and optical properties of a surface as well as their molecular interactions. Adjusting the spacing and morphology of these structures can change how they behave in contact with solids, liquids, biomolecules and how they catalyse certain chemical reactions. Organisms rely on these structures to stay clean, aid communication, and promote or prevent adhesion. The presence of ordered micro- and nanoscale structure appears to the human eye as iridescence created by the selective scatter of certain wavelengths of light.
Dry adhesion
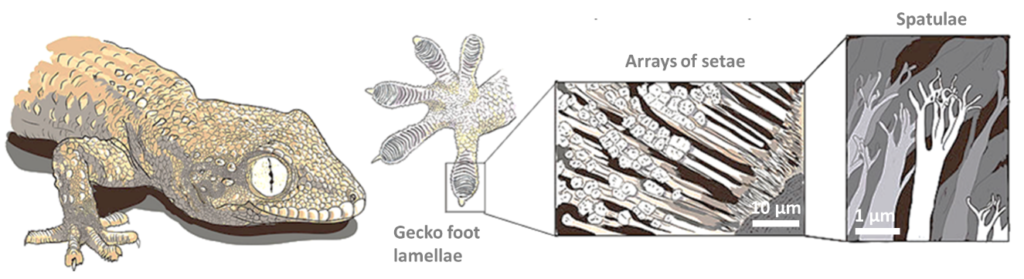
While the exact mechanisms of adhesion differ, the feet of various tree frogs, insects and lizards rely on nanoscale and microscale structure to cling to and climb vertical or inverted surfaces. Perhaps the most famous climbers that rely on adhesion are geckos. Gecko climbing ability comes from millions of hair or setae on their feet which experience Van der Waals interactions with the substrate [1]. Individually the interactions are weak but collectively give the gecko the adhesive force necessary to hold up to four times its own weight. These setae evolved from tiny hair-like growths present on the bodies of all geckos [2]. Generating setae involves lengthening these hairs and splitting the tips to produce micro- and nanoscale hierarchical structures. Curiously, researchers have found that several gecko species developed these adhesive abilities independently when faced with an environment where climbing aided survival, losing them again over time when the environment changed [2].
Figure 2 – Gecko’s secret power by Matteo Gabaglio, Annotation, Order, CC BY-SA 3.0
In the last 20 years, the adhesive strength, reusability and non-fouling properties have attracted increased interest in gecko-inspired adhesives. Manmade micro- and nanoscale hierarchical patterns produced by embossing, casting or roll-to-roll printing have resulted in several tape and patch analogues. As popular as this topic has been, it has not been without its challenges. In addition to difficulties in manufacturing, gecko mimetic adhesives experience poor adhesion to wet and contaminated surfaces [3]. Water disrupts the surface interactions which is also the reason why PTFE (which exhibits weak Van der Waals dispersion forces) is one of few materials that a gecko can’t climb on [4].
Wet adhesion
For wet adhesion it makes more sense to look to water dwelling organisms. Mussels create an adhesive containing a tyrosine residue called DOPA, which has seen increased attention. DOPA and similar coatings are key to allowing nanopattern based adhesives to work in wet conditions. The structure of DOPA allows mussels to form strong and reversible bonds with a variety of substrates [5]. Mussels use this to anchor their pads to rocks and withstand significant punishment from tides and currents. Researchers have so far used DOPA and analogues in an attempt to develop improved surgical adhesives, particularly for amniotic sac repair[6]. Some have combined this with the gecko adhesive above to produce all-purpose hybrids named “Geckel” [7]. These hybrid surfaces consist of a microstructure coated in mussel mimetic adhesive to achieve adhesion in wet or dry conditions. As with any novel technology, achieving a robust product and scalable process has likely limited its implementation. Alternative adhesive-free-adhesives for wet conditions look to the octopus for inspiration. Although an octopus sucker is far larger than the other features we have discussed, its design has been the inspiration for many micro- and nanoscale mimics. Octopodes use suckers as muscular-hydrostats where the internal volume is increased to generate low pressure (≤ 2.7 bar below ambient pressure when submerged) [8]. The octopus vulgaris differs from other species in that it utilises a ball in cup morphology to maintain adhesion and resist shear [9]. Its unique morphology creates two regions of low pressure with the ball protrusion sealing the two volumes and mechanically locking the sucker configuration.
Figure 3 A.) Suckers of octopus by Steve Lodefink, Suckers of octopus by Steve Lodefink, CC BY 2.0. B.) Illustration of sucker adhesion mechanism of Octopus vulgaris.
Octopus mimetic surfaces produced by vacuum casting use microscale suction cups (~ 100 μm) with a similar ball in cup morphology to generate suction [10]. This approach has seen some applications targeting skin but so far appears limited to working on flat surfaces and generating relatively weak vacuums. Some commercial materials such as REGABOND micro-suction foam are aimed for the general consumer market and work on a similar principle [11].
Repulsion
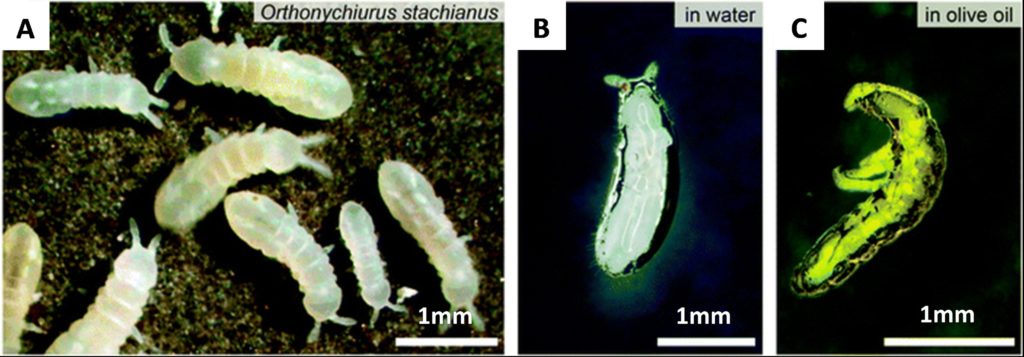
Some plants use micro- and nanoscale texture for an alternative purpose, the “lotus effect” being the most famous example. The lotus effect arises from the ability of micro- and nanostructures to amplify the natural tendency of a surface, making hydrophobic materials superhydrophobic. A lotus leaf has arrays of hydrophobic waxy hierarchical micropillars on its surface [12]. The high roughness and low contact area of these pillars forces water droplets to adopt a Cassie-Baxter state where air is trapped below the fluid meniscus. To reduce the Gibbs free energy of the system the water droplets adopt a highly rounded shape. This allows them to slide off and pick up dirt in the process, keeping the leaves free of debris. The springtail takes this effect further with a cuticle that has a re-entrant or overhanging surface structure [13]. These structures resemble nanoscale mushrooms which pin the fluid line to prevent even low surface tension fluids from fully wetting the surface in what is referred to as oleophobicity. The springtail uses this for survival creating an air trap around its body when submerged. Both superhydrophobicity and oleophobicity are found in industry, often finding use in semipermeable membranes and self-cleaning coatings. The surface energy and morphology of the of the coating material dictate the degree of nonwetting. These structures are still vulnerable in high pressure applications where the structures or the film of air can collapse.
Figure 4 – The springtail cuticle has been used as inspiration for manmade re-entrant omniphobic surfaces A.) Springtails. B.) Springtail submerged in water. C.) Springtail submerged in oil. Scale bars: 1 mm. Image from R. Hensel et al. [13], CC BY-NC 3.0.
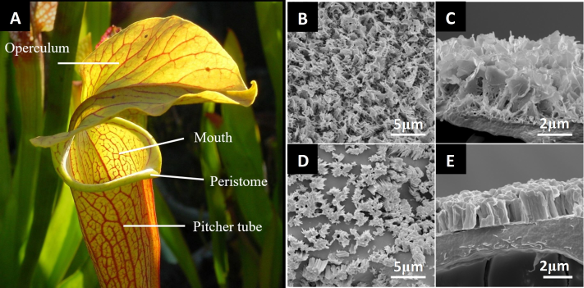
A very different, and potentially more robust approach is used by the pitcher plant. In these plants a microporous surface is used to retain a lubricating fluid film. The films are created when water or nectar becomes locked into microscale textures in the surface of the plant creating a continuous layer of lubrication. The film is immiscible in the oil on the insect’s feet resulting in a surface that easily shears away on contact and very low friction. Unlucky insects which land on the plant’s lip end up sliding down into the plants digestive fluid to become a snack. The film is replenished by capillary effects which redistribute fluid across the film surface. The advantage of this arrangement is the immiscible fluid is incompressible unlike the air used by the lotus leaf and allowing it to serve in higher pressure applications.
Figure 5 A.) Sarracenia pitcher anatomy by Noah Elhardt, Sarracenia pitcher anatomy basic, marked as public domain. B-E.) Microstructure of N. gracilis waxy surfaces. Scale bars shown. Image from Bauer et al. [14], CC BY 4.0.
Pitcher plant mimetic surfaces have been named “slippery liquid-infused porous surface(s)” or SLIPS. These surfaces can be tailored and often use a lubricant which is immiscible in the target substance. The porous substrate consists of open interconnected pores to retain the lubricating fluid. While evidence of industrial application is limited, it is a promising route to stain-resistant coatings for optics.
Optical effects
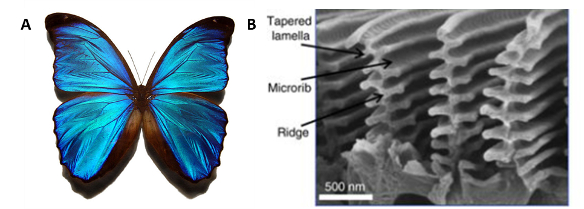
Certain organisms use micro and nanostructures to produce iridescence that makes the rest of the animal kingdom pale by comparison. While many rely on chemicals for coloration, using microscale structures is called structural coloration or physical colour. Butterflies use this effect for visual communication to find mates or scare away would-be predators. The most famous example is the Morpho butterfly native to Latin America [15]. The Morpho is an underwhelming (but well hidden) shade of brown with its wings closed but a bright iridescent blue when they open. The blue iridescence comes from tiny chitin gratings on the surface of a butterfly’s wings [3]. The layering of these structures causes diffraction and constructive interference of visible light waves according to Bragg’s law, producing the visual perception of a very intense colour [17]. The angle at which the butterfly is observed changes the colour we perceive the wings to be, shifting from blue to copper when viewed at an angle. The papilionidae family of butterflies use similar architectures combined with fluorophores to harvest NIR light to create luminescence [18].
Figure 6 – A.) Blue morpho butterfly by Gregory Phillips, Blue morpho butterfly, CC BY-SA 3.0. B.) Nanoscale Structures on a Blue Morpho Butterfly Wing Image from Potyrailo et al. [19], CC BY 4.0.
The unique physical, chemical, and optical properties of these structures have led to interest in several industries. Extensive research and development efforts have gone into mimicking these effects for energy harvesting, sensing and photocatalysis [19]. For medical applications they have a role to play in optical biosensing. By coating a grating in environmentally responsive molecules or hydrogels an optical indicator can be constructed. Structures such as this have been coined hydrogel-actuated integrated responsive systems (HAIRS)[20].
We hope that this has been an interesting read. The next edition will discuss the practicality of fabricating these structures, and their suitability for parts used in medical devices.
If you have any questions about micro engineering and smart surfaces, please do not hesitate to get in touch or find me on LinkedIn.
Bibliography
[1] K. Autumn and N. Gravish, “Gecko adhesion: evolutionary nanotechnology,” Philos. Trans. R. Soc. A Math. Phys. Eng. Sci., vol. 366, no. 1870, p. 1575 LP-1590, May 2008.
[2] T. Gamble, E. Greenbaum, T. R. Jackman, A. P. Russell, and A. M. Bauer, “Repeated origin and loss of adhesive toepads in Geckos,” PLoS One, vol. 7, no. 6, 2012.
[3] A. Y. Stark, T. W. Sullivan, and P. H. Niewiarowski, “The effect of surface water and wetting on gecko adhesion,” J. Exp. Biol., vol. 215, no. 17, p. 3080 LP-3086, Sep. 2012.
[4] A. Y. Stark et al., “Adhesive interactions of geckos with wet and dry fluoropolymer substrates,” J. R. Soc. Interface, vol. 12, no. 108, p. 20150464, Jul. 2015.
[5] J. H. Waite, “Mussel adhesion – essential footwork,” J. Exp. Biol., vol. 220, no. 4, p. 517 LP-530, Feb. 2017.
[6] M. Perrini, D. Barrett, N. Ochsenbein-Koelble, R. Zimmermann, P. Messersmith, and M. Ehrbar, “A comparative investigation of mussel-mimetic sealants for fetal membrane repair,” J. Mech. Behav. Biomed. Mater., vol. 58, pp. 57–64, 2016.
[7] H. Lee, B. P. Lee, and P. B. Messersmith, “A reversible wet/dry adhesive inspired by mussels and geckos,” Nature, vol. 448, p. 338, Jul. 2007.
[8] J. J. Wilker, “How to suck like an octopus,” Nature, vol. 546, p. 358, Jun. 2017.
[9] F. Tramacere, L. Beccai, M. Kuba, A. Gozzi, A. Bifone, and B. Mazzolai, “The Morphology and Adhesion Mechanism of Octopus vulgaris Suckers,” PLoS One, vol. 8, no. 6, p. e65074, Jun. 2013.
[10] S. Baik, D. Wan Kim, Y. Park, T.-J. Lee, S. Ho Bhang, and C. Pang, “A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi,” Nature, vol. 546, pp. 396–400, 2017.
[11] “materialdistrict.com.” [Online]. Available: https://materialdistrict.com/material/regabond-s. [Accessed: 05-Sep-2018].
[12] T. Darmanin and F. Guittard, “Superhydrophobic and superoleophobic properties in nature,” Mater. Today, vol. 18, no. 5, pp. 273–285, 2015.
[13] R. Hensel, C. Neinhuis, and C. Werner, “The springtail cuticle as a blueprint for omniphobic surfaces,” Chem. Soc. Rev., vol. 45, no. 2, pp. 323–341, 2016.
[14] U. Bauer, B. Di Giusto, J. Skepper, T. U. Grafe, and W. Federle, “With a Flick of the Lid: A Novel Trapping Mechanism in Nepenthes gracilis Pitcher Plants,” PLoS One, vol. 7, no. 6, p. e38951, Jun. 2012.
[15] Y. Ding, S. Xu, and Z. L. Wang, “Structural colors from Morpho peleides butterfly wing scales,” J. Appl. Phys., vol. 106, no. 7, pp. 1–6, 2009.
[16] R. Yan et al., “Bio-inspired Plasmonic Nanoarchitectured Hybrid System Towards Enhanced Far Red-to-Near Infrared Solar Photocatalysis,” Sci. Rep., vol. 6, no. December 2015, pp. 1–11, 2016.
[17] S. Zhang and Y. Chen, “Nanofabrication and coloration study of artificial Morpho butterfly wings with aligned lamellae layers,” Sci. Rep., vol. 5, pp. 1–10, 2015.
[18] E. Van Hooijdonk, C. Vandenbem, S. Berthier, and J. P. Vigneron, “Bi-functional photonic structure in the Papilio nireus (Papilionidae): modeling by scattering-matrix optical simulations,” Opt. Express, vol. 20, no. 20, p. 22001, 2012.
[19] R. A. Potyrailo et al., “Towards outperforming conventional sensor arrays with fabricated individual photonic vapour sensors inspired by Morpho butterflies,” Nat. Commun., vol. 6, p. 7959, Sep. 2015.
[20] J. M. J. den. Toonder and P. R. Onck, “Artificial cilia.” Royal Society of Chemistry, Cambridge, 2013.