Design controls in medical device development: Ensuring safety and quality
20 June 2023
The development of medical devices is a complex and highly regulated process that requires meticulous attention to detail and a focus on ensuring patient safety. Each project that Springboard works on follow strict design controls ensuring that the products we work on meet these regulations.
Design controls play a crucial role in this process, providing a systematic approach to manage risks, design requirements, and verification and validation activities. The internationally recognized standard that outlines the requirements for design controls in the medical device industry is ISO 13485, which in this aspect is in alignment with the U.S. rules for design controls in FDA 21 CFR Part 820.30. In this blog, we will explore the importance of design controls and discuss the key elements of ISO 13485 related to medical device development.
Why design controls matter:
- Design controls are essential for medical device development as they ensure that devices are designed, developed, and manufactured in a consistent and controlled manner. By implementing robust design controls, developers can:
- Enhance patient safety: Design controls enable you to identify and mitigate risks associated with the use of medical devices, reducing the potential for harm to patients and users.
- Improve product quality: Effective design controls help to establish and maintain quality throughout the development process, resulting in reliable and consistent devices that meet user needs and regulatory requirements.
- Facilitate regulatory compliance: Compliance with design control requirements is a critical aspect of obtaining and maintaining regulatory approvals and certifications, such as the CE mark or FDA clearance.
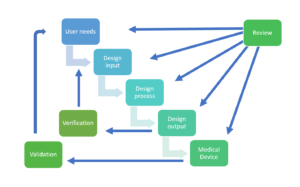
Design control process
Key elements of ISO 13485 design controls:
- Design and development planning:
- ISO 13485 emphasizes the importance of planning the design and development process, which includes defining design inputs, establishing design and development stages, and determining the necessary resources and responsibilities. · Design Inputs:
- Design inputs specify the requirements that a medical device must meet to address the needs of users, patients, and other stakeholders. These inputs include performance requirements, safety and regulatory requirements, and user needs.
- Design outputs:
- Design outputs are the tangible results of the design process, such as drawings, specifications, and manufacturing instructions. ISO 13485 requires that these outputs be documented and reviewed to ensure they meet the design inputs and are suitable for manufacturing.
- Design reviews:
- Regular design reviews are conducted throughout the development process to assess the progress, identify issues, and verify that the design outputs meet the design inputs. These reviews involve cross-functional teams and provide opportunities for feedback and improvement.
- Design verification and validation:
- Design verification confirms that the device design meets the specified design inputs, while validation ensures that the device meets the needs of the intended users and performs safely and effectively within its intended use environment. ISO 13485 provides guidance on establishing appropriate verification and validation activities.
- Design transfer to manufacture:
- Design transfer involves the transfer of the design to manufacturing, ensuring that all necessary information and documentation are provided to facilitate production. ISO 13485 emphasizes the importance of effective communication and collaboration between design and manufacturing teams during this phase.
- Design changes:
- The standard requires a systematic approach to managing design changes, including documenting, reviewing and approving any modifications made to the device design. All change reviews must evaluate the effect on existing products, risk management and product realisation activities.
- Design and development file
- The conformity to these design and development requirements needs to be recorded for each medical device. This can be in a dedicated file or as reference to existing records. In the US, these files are known as Design History Files.
Design controls are an integral part of medical device development, contributing to the safety, quality, and regulatory compliance of devices. Springboard adheres to ISO 13485 guidelines for design controls that provide a comprehensive framework to manage the design and development process effectively. By following these controls, we can mitigate risks, improve product quality, and ultimately deliver safe and effective medical devices to improve patient care.
Contact us today to find out how we can help.
Written by Thom Wyatt, Principal Engineer