ISO just moved the goalposts for injection devices
17 May 2022
Introduction
The ISO 11608 family of standards for Needle-based Injection System (NISs) was recently updated for the first time since 2014. Many of the changes make the standards better suited to the newer types of NISs which are becoming increasingly common, such as On Body Delivery Systems (OBDSs) and NISs containing electronics.
The 11608 family
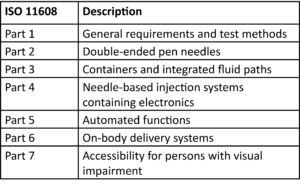
Table 1: Description of the parts of the family
11608-1 is the parent standard to the 11608 family and covers the general requirements and test methods for NISs. Part of the update has been to relocate some of the content which was previously in 11608-1 to other standards in the family, for example requirements relating to electronic devices such as EMC are now solely within ISO11608-4.Table 1: Description of the parts of the family
11608-3 has been updated to include integrated fluid paths as well as finished containers, covering rigid needles, soft cannulas, and any fluid line connections. 11608-4 has also been expanded to cover a wider range of functions with electronic NISs, again reflecting the development within the industry and the increasing amounts of functionality.
11608-6 covers OBDSs, which are not to be confused with infusion pumps or insulin patch pumps. In infusion pumps or insulin patch pumps the rate of delivery of the medicinal product is important to its clinical function, whereas an OBDS is one which delivers a discrete volume, or “bolus”, in a time which is governed by tolerability and convenience to the user.
“Primary functions”
New to the 2022 version of 11608 is the introduction of the term “primary functions”. This replaces the term “essential performance” (which is also used in IEC 61010-1 for laboratory equipment) and is defined as:
A “function or operation of the needle-based injection system which, if it does not perform to specifications during use, would directly result in a failure to accurately deliver the medicinal product via the correct route and/or directly result in unacceptable harm to the patient”
When it comes to verification of requirements identified as primary functions, the same approach and assessment criteria set out for dose accuracy should be applied.
Whilst the 2014 version of 11608-1 referenced ISO 14971 and IEC 62366 for Risk Management and Usability Engineering respectively, these sections are expanded in the 2022 version and explicitly state that risk management tools should be applied to the primary functions defined above.
What does this all mean?
In general, the updates are unlikely to mean significant changes to the way in which NISs are verified, but the update provides a welcome improvement to the structure to suit modern classes of device. If you are developing an On Body Delivery System or an electronic NIS then updates to parts 6 and 4 should be reviewed, along with the overall requirements in part 1. The wording around primary functions should also be noted for the purposes of documentation; primary function includes situations where the device could directly cause harm to the patient, which would be considered “basic safety” under ISO 61010, as well as the clinical function of the device to correctly deliver the medicinal product.
If you are concerned about how the changes to the ISO standard might affect your device development, then please do get in touch – Springboard have years of experience in developing Needle-based Injection Systems as well as other types of drug delivery devices.
Find out more about how our approach could help you successfully get your device to market here.
-Adam Nightingale


