Best Practices Handling Material Changes For Medical Devices
26 March 2024
Medical device companies are heavily reliant on injection-molded plastic components, which might be produced either in-house or through a contract manufacturer. The properties of the polymers used can be intrinsically linked to the behavior and performance of a device, which makes understanding and controlling these properties a business-critical matter.
Despite the best intentions of procurement, changes in the availability of material grades can be imposed by suppliers. This might be a result of new regulations or simply strategic changes on the part of the supplier. A long-running device may be subject to multiple changes over its lifetime. Any business relying on materials and their properties should have a process for anticipating, controlling, and mitigating the effects of material changes in order to:
- manage and reduce risk,
- avoid interrupting production runs,
- detect and head off problems early on, and
- understand how to control batch-to-batch variation.
Material changes can pose an existential risk to medical device lines, and should be taken seriously, planned for, and their effects mitigated against.
Won’t The Supplier Take Care Of It?
Most likely, you’ve signed some sort of agreement with your material supplier, guaranteeing an uninterrupted supply of material for the lifetime of your product. Doesn’t that mean your material supplier has a duty to supply a suitable alternative?
In an ideal world, yes. But most suppliers are only willing to guarantee what’s on the datasheet, and that may be only a small subset of the material’s properties that can influence the behavior of a device. An “identical” substitute material grade may perform completely differently when the full range of properties required by a device are taken into account.
So Which Properties Are You Interested In?
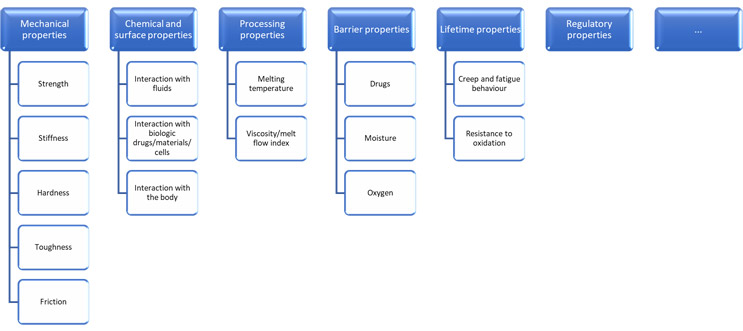
The process should start by establishing which material properties are critical to quality. Depending on the device, these could be selected from a wide range of material characteristics – not all of which will be on the datasheet.
Click on figure to enlarge.
Some of these are in turn determined by more basic structural properties of the polymers themselves, such as:
- molecular weight distribution
- tacticity
- copolymer ratios
- crystallinity
- and more.
Many of these properties will be fixed in the resin as supplied, while others are affected by melt processing and other manufacturing processes.
What Should Be In The Material Change Toolkit?
There is a huge variety of techniques available for characterizing materials, and a combination of application-specific understanding and specialist analytical insight is vital for choosing the most appropriate techniques. A well-equipped toolkit for handling change must include means of evaluating each property of interest:
- Mechanical properties can be evaluated using mechanical testing methods, including uniaxial tensile testing, indentation, and various forms of notch toughness testing.
- Chemical and surface properties can be evaluated using a wide variety of analytical techniques — FTIR, Raman, SIMS, for example — as well as through biocompatibility test methods as appropriate.
- Processing properties can be evaluated using thermal techniques, including melt flow index testing, differential scanning calorimetry, and thermogravimetric analysis.
- Barrier properties can be evaluated using permeability testing.
- Lifetime properties can be evaluated more quickly using accelerated aging.
- Polymer structure properties can be evaluated using thermal techniques, gel permeation chromatography (molecular weight), mass spectrometry (copolymer ratios), X-ray diffraction (crystallinity, tacticity), and more.
When Should You Start Thinking About This?
In order to apply these tools, it’s important to understand what range of measurements are acceptable, and this starts right back at the design stage. Functional material properties should be used and specified consciously and deliberately at the design stage, rather than being treated as incidental to the design process.
A robust technical requirements specification should include tolerances on all critical-to-quality parameters – including material properties. This can be challenging, as materials science is not always part of design language.
It’s also vital to understand what “normal” looks like, which means you need to characterize your existing material fully before any change is imposed, and before it’s in short supply. This has additional benefits in building your understanding of batch-to-batch variation and process control in general.
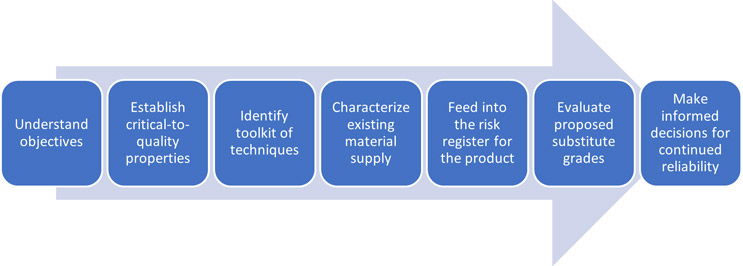
What Does A Good Material Change Process Look Like?
Click on figure to enlarge.
A well-balanced material change process should be established well before it’s needed, and should be part of a product’s risk register. Critical-to-quality properties should be identified and tracked from the design stage through to manufacture. The tools needed to fully characterize these properties should be available and fit for purpose. Existing material stocks should be thoroughly characterized, rather than relying on datasheets or information provided by material suppliers.
All of this gives you the best possible baseline to rapidly and comprehensively evaluate a material change and ensure that the performance and reliability of your device are not compromised.
Get in touch today if you need assistance with your materials strategy.
Written by Philip Howie, Ph.D. Principal Materials Scientist