The next generation of microneedles; harnessing a billion years of evolution
12 April 2023
In this article, we consider examples of nature’s solutions to four challenges faced by microneedles for drug delivery. For each challenge, the authors explain how a natural organism has tackled it and what lessons may be directly applied to microneedle design.
The problem with needles
Many people are afraid of needles, and a significant number experience such an aversion to needles that they will avoid seeking medical care (5). The invention of a needle so thin and short that it is barely visible to the unaided eye, called a microneedle, would be welcomed by the needle-phobic, and would bring other benefits such as near-painless injections, easy self-administration and the freedom to dispose of the device with a negligible sharps risk. Further, such a device would be ideally suited to the administration of drugs into the skin, which may advantageously induce a greater immune response when administering vaccines (4) and avoids first- pass metabolism of the drug (6). These benefits are the driving force behind the predicted growth of the microneedle transdermal drug delivery market from 5.6 billion USD in 2020 to 8 billion USD by 2028 (7).
R&D efforts are well underway
The motivating factors above have resulted in the investment of great effort into the development of microneedle drug delivery devices (1). Most are one of four types (solid, coated, hollow or dissolving microneedles), but many less-common variants have been investigated.
Only a small number of therapeutic microneedle products have made it to market so far (8), such as the BD Soluvia® injector and the NanoPass Micronjet®, which can deliver consistent and painless intradermal injections of drugs and vaccines (9). The Sanofi IDflu®/Intanza® was also commercialised worldwide for the delivery of influenza vaccines (9).
A growing number of microneedle technologies for the delivery of drugs and vaccines are in clinical trials. For example, PharmaTher and TSRL are co-developing a microneedle patch to deliver psychedelics and antivirals (10); Radius Health and Kindeva Drug Delivery are evaluating a solid microneedle device for osteoporosis (11); and Micron Biomedical are running a clinical trial for a measles-rubella vaccine administered through microneedles (12).
Many challenges remain
The novelty of microneedles bring many challenges, and despite good progress some of these remain to be overcome before they see widespread use for drug delivery. The spectrum of challenges has been well documented elsewhere (1), and ranges from difficulties in reproducible manufacturing to ensuring reliable delivery of a complete dose.
The first step to solving most problems is to consider whether they may have been solved already. Even if no prior solutions are a perfect fit, some may be readily adaptable to the present challenge, or serve as inspiration to resolve the issue in a drastically different way. An ever-bountiful source of solutions and inspiration is to be found in the natural world, in which ruthless evolution over millions of years has created countless necessarily elegant technical solutions to many problems.
In this article we consider examples of nature’s solutions to four challenges faced by microneedles today. For each challenge, we explain how the relevant organism has tackled it, and what lessons may be directly applied to microneedle design.
Challenge: Penetration
Through natural selection, mosquitoes have developed a sophisticated apparatus to effectively and painlessly penetrate the skin of animals and humans to feed on their blood (13). This apparatus consists of a bundle, or fascicle, of fine tools called stylets that are housed within the mosquito’s proboscis. Together, these stylets comprise a natural microneedle 2 mm long and a mere 20 µm in diameter (2). But how does the mosquito use such a delicate structure to penetrate the skin? The answer is partly in a mechanical feature, and partly in how the fascicle is deployed.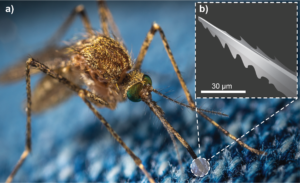
Figure 1: A) A mosquito uses specialised microneedles in its mouthparts to painlessly bite through animal skin. (B) An illustration of a mosquito fascicle, revealing a micro-serrated edge used to cut through skin tissue.
First, the structure has micro-serrations that reduce the contact area between the fascicle and skin tissue, which significantly lowers insertion forces (Figure 1). Indeed, studies have found that a living mosquito fascicle requires an insertion force of around 16 µN, which is approximately one thousandth of the force required to insert synthetic ultra-sharp microneedles (14).
Second, mosquitoes deploy the fascicle in a way that makes good use of those serrations. Having probed for an ideal spot to penetrate, the mosquito anchors its fascicle onto the skin, then oscillates its head back and forth in a sawing motion to drive the fascicle through the skin. Even this sawing motion is carefully modulated to optimise penetration, starting at 17 Hz and slowing to 6 Hz as the needle is driven deeper (2). Using FEM simulation (15) and skin-penetration models (16), researchers have already started to develop mosquito-inspired microneedles that have serrated tips and use cooperative vibration to reduce skin-penetration forces. To our knowledge, such features have yet to be exploited clinically.
Challenge: Fracture
Not all microneedles in nature are designed to be painless. The caterpillar Parasa consocia is covered in hollow microneedle spines that pierce any would-be predators and inject a pain-inducing venom. They feature several intriguing microstructures, including a tapered geometry, a side-open tip, a barbed surface, and neck-like grooves; but perhaps the most intriguing feature is a subtle one.
Because they are so fine, microneedles can be particularly susceptible to bending and compressive buckling failure. Whereas the mosquito’s fascicle uses serration and vibration to avoid buckling, the caterpillar’s spine employs a different strategy. The magnitude of buckling is directly related to the Young’s modulus of the material (18), and both SEM imaging and atomic force spectroscopy have revealed that the Young’s modulus of each caterpillar spine increases progressively from base to tip (17) (Figure 2). This gradated material property, together with the geometry of the spine, means that the spines can withstand compressive and bending of up to 3 mN before fracture, which is an order of magnitude greater than the insertion forces required for these ultra-sharp needles to penetrate animal skin (17). Applying these lessons to synthetic microneedles may make them more mechanically robust to a common mode of failure.
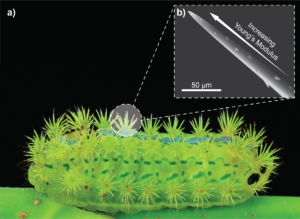
Figure 2: A) A Parasa conoscia caterpillar with venomous spines robust against mechanical failure. (B) Illustration of the microstructure of the spines, in which Young’s modulus increases towards the tip to prevent buckling and bending under compressive stress.
Challenge: Adhesion
Some medical conditions require not a single, rapid injection but rather gradual release of a therapeutic agent over time, and microneedle patches are a promising vehicle for such sustained release (22). However, it can be difficult to ensure that a microneedle patch remains attached to the tissue, especially in regions of skin that are subject to large motions. Here, too, one may draw inspiration from biological organisms.
Recently, a microneedle array was developed that was designed to adhere to the user using two different mechanisms, each inspired by a different animal (19) (Figure 3). First, each microneedle was surrounded by microscale suction cups, which were modelled after the suckers on octopus tentacles. Second, the suction cups and microneedles were made of a poly-dopamine hydrogel inspired by the composition of the filaments (called byssi) that mussels use to attach to a range of surfaces. The improved adhesion thus achieved, without the use of medical adhesive tape, could make microneedle patches a more viable option for the sustained release of drugs to mobile parts of the body, such as on knee joints to deliver therapeutics for osteoarthritis (23).

Figure 3: Array of microneedles (centre) with bio-inspired adhesive abilities. Each microneedle is surrounded by suction cups inspired by octopus tentacle suckers (left), and composed of an adhesive poly-dopamine hydrogel inspired by the filaments used by mussels to adhere to rocks (right).
Challenge: Delivery
It is generally difficult to ensure that a microneedle system delivers a complete dose of a liquid drug formulation, especially if the viscosity and surface energy of the formulation have not been carefully engineered for use with microneedles. Fortunately, there already exists a solution that delivers a liquid formulation rapidly and reliably into animal tissue.
In a collection of species known as rear-fanged snakes, some of their fangs are not hollow, but instead feature a groove that runs along the fang’s surface (Figure 4). When the snake bites into an animal, venom runs down the capillary channel formed between the groove and the unfortunate animal’s tissue. Understanding how this flow is driven by surface tension allowed researchers to exploit the same effect, and further optimise it by designing synthetic microneedle “fangs” with multiple grooves on each (24). The use of solid rather than hollow microneedles for the delivery of liquid drugs improves the mechanical strength of the needles and simplifies their manufacture. The technology shows promise for the delivery of fast vaccinations, being capable of inducing a robust immune response from a single administration (24).

Figure 4: (A) Rear-fanged snake with (B) mono-grooved fangs to aid delivery of venom to prey. (C) Snake fang multi-grooved solid microneedles, optimised for delivery of liquid formulations.
Conclusion
The development of a novel, successful medical device is typically a hugely ambitious undertaking. A concept that seems promising at first may fall at any number of hurdles on its way to launch: it may be let down by material properties, variability in manufacturing, reaction to sterilisation, or subtle design issues that only manifest in late-stage testing. Leveraging existing solutions to closely-related problems elsewhere, whether in industry or nature, can dramatically speed up product development. Technology scouting is an essential part of rapid innovation: by avoiding reinventing the wheel, it frees up resources for investment where absolutely necessary, and reduces the time to market for solutions that patients urgently need.
Written by Ethan Miller and Gabriel Villar and featured in OnDrug Delivery
- Microneedles in drug delivery: progress and challenges. Avcil, Muhammet and Çelik, Ayhan. 2021, Micromachines, p. 1321.
- Microneedle, bio-microneedle and bio-inspired microneedle: a review. Ma, Guojun and Wu, Chengwei. 2017, J. Control. Release, pp. 11-23.
- Bio-inspired barbed microneedle for skin adhesion with interlocking mechanics. Tran, Le-Giang, Nguyen, Thanh-Qua and Park, Woo-Tae. 2019, IEEE 32nd Conference on MEMS, pp. 547-550.
- Recent advances of microneedles used towards stimuli-responsive drug delivery, disease theranostics, and bioinspired applications. Yang, Jingbo, et al. 2021, Chem. Eng. J., p. 130561.
- Considering needle phobia among adult patients during mass Covid-19 vaccinations. Love, Ashley S. and Love, Robert J. 2021, J. Primary Care & Comm. Health, pp. 1-4.
- A comprehensive review of microneedles: types, materials, processes, characterizations and applications. Aldawood, Faisal Khaled, Andar, Abhay and Desai, Salil. 2021, Polymers, p. 2815.
- Grand View Research. Microneedle drug delivery systems market size, share & trends analysis report by type (hollow, dissolving, solid, coated), by material (metal, silicon, polymer), by application, by region, and segment forecasts. Grand View Research. [Online] 29 November 2022. https://www.grandviewresearch.com/industry-analysis/microneedle-drug-delivery-systems-market-report.
- Microneedle Array: Applications, Recent Advances, and Clinical Pertinence in Transdermal Drug Delivery. Jitu Halder, Sudhanshu Gupta, Rakhi Kumari, Ghanshyam Das Gupta, Vineet Kumar Rai. 2020, Journal of Pharmaceutical Innovation, pp. -.
- Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commerical development. Eneko Larraneta, Rebecca E.M.Lutton, A.David Woolfson, Ryan F.Donnelly. 2016, Material Sceince and Engineering R, pp. 1-32.
- Ltd, PharmaTher Holdings. PharmaTher and TSRL enter into co-development agreement for microneedle patch delivery technology for psychedelics and antivirals. GlobeNewsWire. [Online] 01 June 2021. https://www.globenewswire.com/news-release/2021/06/01/2239417/0/en/PharmaTher-and-TSRL-Enter-into-Co-Development-Agreement-For-Microneedle-Patch-Delivery-Technology-for-Psychedelics-and-Antivirals.html.
- Phase 1b Evaluation of abaloparatide solid microstructured transdermal system (Abaloparatide‑sMTS) in postmenopausal women with low bone mineral density. Miller, Paul D., et al. 2021, Clin. Drug Invest., pp. 277-285.
- Health, National Institutes of. Measles and rubella vaccine microneedle patch Phase 1-2 age de-escalation trial. ClinicalTrials.gov. [Online] 20 October 2021. https://clinicaltrials.gov/ct2/show/NCT04394689.
- The effects of geometry on skin penetration and failure of polymer microneedles. Shaun D. Gittard, Bo Chen, Huadong Xu, Aleksandr Ovsianikov, Boris N. Chichkov,Nancy A. Monteiro-Riviere, Roger J. Narayan. 2012, Journal of Adhesion Science and Technology, pp. 227-243.
- Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. Gasser, Thomas Christian, Holzapfel, Gerhard and Griss, Patrick. s.l. : IEEE Xplore, 2008, IEEE Xplore, pp. 1429-1440.
- Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito’s proboscis. Seiji Aoyagi, Hayato Izumi, Mitsuo Fukuda. 2008, Sensors and Actuators A: Physical, pp. 20-28.
- Realistic imitation of mosquito’s proboscis: Electrochemically etched sharp and jagged needles and their cooperative inserting motion. Hayato Izumi, Masato Suzuki, Seiji Aoyagi, Tsutomu Kanzaki. 2011, Sensors and Actuators A: Physical, pp. 115-123.
- Biomechanical Property of a Natural Microneedle: The Caterpillar Spine . G. J. Ma, L. T. Shi, C. W. Wu. 2011, Journal of Medical Devices, p. 034502.
- Buckling analysis of polymer microneedle for transdermal drug delivery. C. Radhika, B.K Gnanavel. 2020, Materials Today: Proceedings, pp. 3538-3541.
- Bioinspired adhesive and antibacterial microneedles for versatile transdermal drug delivery. Zhang, Xiaoxuan, et al. 2020, Research.
- Picchiottino, Diane. Brown and black dragon in water. Unsplash: 2021.
- Castille, Andy. Black and red ladybug on white textile. Unsplash: 2020.
- Why drug delivery is the key to new medicines. May, Mike. 2022, Nature Medicine, pp. 1100-1102.
- Strategy for osteoarthritis therapy: Improved the delivery of triptolide using liposome-loaded dissolving microneedle arrays. Zhou, Ping, et al. 2021, International Journal of Pharmaceutics, p. 121211.
- Snake fang-inspired stamping patch for transdermal delivery of liquid formulations. Bae, W. G., et al. 2019, Science Translational Medicine, p. eaaw3329.
- Microneedles: a potential strategy in transdermal delivery and application in the management of psoriasis. Zhao, Zihan, Chen, Youdong and Shi, Yuling. 2020, RSC Adv., p. 14040.
- Microneedle for transdermal drug delivery: current trends and fabrication. Jung, Jae Hwan and Jin, Sung Giu. 2021, J. Pharm. Invest., pp. 503-517.
- Biting Innovations of Mosquito-Based Biomaterials and Medical Devices. Angela R. Dixon, Isabelle Vondra. 2022, Materials, p. 4587.
- Pixabay. Diamond back rattle snake. Pexels, Glasgow: 2016.