Shielded Therapeutics: Could Polymeric Nanoparticles Safeguard a New Generation of Inhaled Drugs?
30 April 2025
Polymeric nanoparticles (PNPs) represent a groundbreaking advancement at the cutting edge of pulmonary drug delivery, holding great promise for overcoming the key challenges in respiratory medicine. Vital therapeutics such as small molecules, proteins, and nucleic acids could be encapsulated in engineered nanoscale vehicles to evade the natural defences that protect the critical area of our respiratory system.
PNPs offer significant advantages over the more commonly utilised lipid nanoparticles (LNPs) for drug delivery, which are at a more advanced stage across the industry – for example Translate Bio’s MRT5005, which is an inhaled LNP carrying mRNA administered by nebulisation that is currently in Phase 1/2 clinical trials.1 Compared to LNPs, PNPs provide greater stability, resisting enzymatic degradation and fusion in biological environments.2 They also provide greater methods of controlled drug release and can be functionalised for targeted delivery. With good biocompatibility, reduced immunogenicity, and improved mucus penetration, PNPs could present a more effective alternative to LNPs for pulmonary drug delivery.
Breaching the fortress
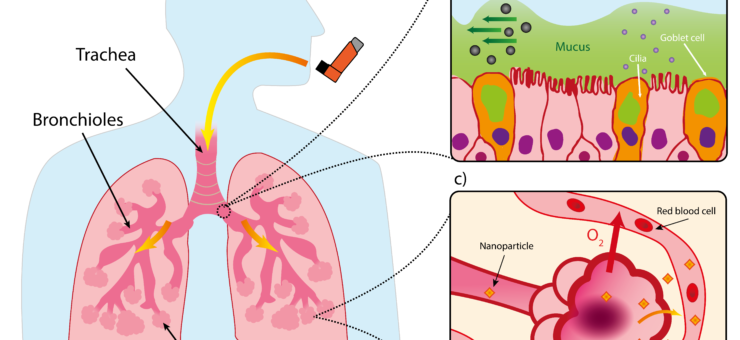
Drugs intended for systemic effects must reach the alveoli, the deep lung region with a huge surface area totalling an estimated 140 m2 and a rich blood supply, both of which facilitate gas exchange. To protect this vital function, our lungs have a unique and multilayered defence system that filters out any foreign invaders. Particles larger than 5 μm are subject to the mucociliary escalator, the self-clearing mechanism consisting of the mucous layer and cilia underneath as shown in Figure 1.
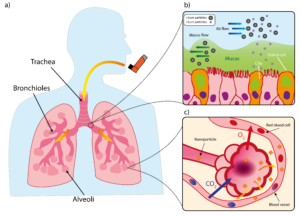
Figure 1: Structure of lung and mucociliary escalator. (a) Simplified illustration of lung. b) Illustration of mucociliary escalator clearing larger particles from airways. (c) Diagram of alveoli with large surface area to facilitate gaseous exchange but also provide route for systemic delivery of nanoparticles via blood stream
While they may be technically able to ‘reach’ the alveolar region due to their size, submicron particles (i.e. the <1000 nm sized particles we discussing in this article) have such low inertia that they would simply be exhaled.3 PNPs therefore require a supporting medium to achieve particle sizes in the 1 to 5 μm which the correct range to deposit in the deep lung via sedimentation,4 and the closer the particles can get towards the smaller end of that scale, the better for alveolar access.5 Inhalation devices therefore target metrics like a high fine particle fraction (FPF), which is the percentage of aerosolised particles below 5 μm, and a low mass median aerodynamic diameter (MMAD), the representative diameter below which 50% of the particle population of an aerosol lies. PNPs could also have classical respiratory applications where the target may be the bronchi and bronchioles themselves, for example facilitating the delivery of bronchodilators or corticosteroids.
To achieve the right particle size for the application, the inhalation device used for delivery of PNPs must be carefully chosen or designed to ensure that the delivery of the therapeutic does not fall at the final hurdle. Table 1 below shows some potential options for PNP-based delivery devices:
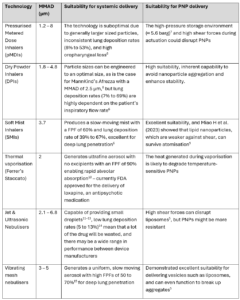
Table 1: Pulmonary delivery devices and their suitability for systemic and PNP delivery
Once the device has delivered the PNP to its intended location in the lung, it can then carry out its key functions, which could be a mixture of:
- Protecting sensitive therapeutics, such as nucleic acids and proteins, from degradation in the harsh respiratory environment and enhance their delivery efficiency. Respiratory mucus contains nucleases and proteases that would quickly degrade them via enzymatic attack.16
- Targeting specific cell types within the lung (e.g. tumor cells) using ligands placed on the shell, for example antibodies or peptides.4
- Providing transport mechanisms to biologics that if unprotected would be immobilised by mucin fibers – PNPs with muco-inert coatings (e.g. PEG) can reduce adhesion for better diffusion through the mucous mesh.17
- Evading clearance by macrophages via phagocytosis by engineering the shell or surrounding matrix of the PNP, thus sidestepping one of the primary functions of our innate immune system. For most microspheres, engulfment via macrophage is something to be avoided, however there are some cases e.g. vaccines where triggering a macrophage response is the aim of the therapy.18
- Improving of drug uptake into the cells via endocytosis-based pathways.4
- Translocating from the alveoli to systemic circulation, removing the need for intravenous administration for a certain drug.4
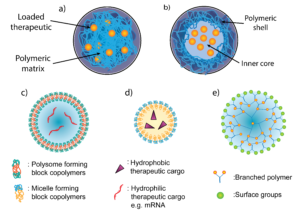
Figure 2: Different structures of PNPs. Two main categories of PNP for pulmonary delivery, (a) nanospheres and (b) nanocapsules. Nanoparticles can also exist in various shapes including (c) polymersomes (d) polymer micelles and (e) dendrimers.
Nanoscale blueprints
As can be seen in Figure 2, there are a wide range of different PNPs which could be used in pulmonary delivery, and much of the literature categorises them into two types19,20:
- Nanocapsules: the drug is usually dissolved in an aqueous or oily reservoir, the release of which is controlled by a polymeric membrane wrapped around it.
- Nanospheres: a continuous matrix system where the drug is retained inside or adsorbed onto its surface.
Within the above categories, PNPs can be divided further into specific shapes, such as21:
- Polymersomes: artificial hollow spheres (vesicles) with membranes built from amphiphilic block copolymers e.g. PEG. They are comparable to liposomes which are vesicles formed from naturally occurring lipids.
- Polymer micelle: also built from amphiphilic block copolymers that instead form nanospheres with a hydrophilic core and hydrophobic coating.
- Dendrimers: Hyperbranched polymers e.g. PEI with complex 3D architectures. Active functional groups on the outer surface can be used to conjugate biomolecules and contrast agents for imaging. They are mostly used for the delivery of nucleic acids or small molecules which can be enclosed within.
There are a wide range of polymer materials that PNPs can be constructed from, examples of which are shown in the table below, along with potential pulmonary applications that are currently under research:
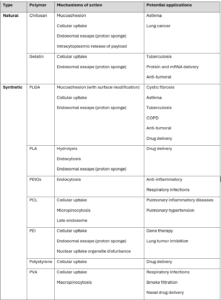
Table 2: PNPs with pulmonary applications22
Semi-automated assembly
A multitude of different processes exist to create the PNPs above, which either involve monomer polymerisation as part of the process, or the dispersion of preformed polymers. Monomer polymerisation techniques, used for polymers such as PLGA, PLA and PCL, usually involve the formation of an emulsion which can go on to self-assemble into nanoparticles.
When using preformed polymers to form PNPs, for example polystyrene, PVK and PMMA, a solvent is normally utilised, and precipitation of nanoparticles can be induced by removal of the solvent by evaporation/diffusion, adding salt or de-solvation. In both categories of PNP formation, surfactants e.g. PVA or crosslinkers can also be used to control size and prevent aggregation, but need to be eliminated from the final product.19
Compared to the lipid nanoparticle (LNP) processes used for COVID-19 vaccines, which used microfluidics at small scale and scaled up by using confined impinging jet turbulent mixers,23 a larger amount of steps are required to synthesise PNPs and therefore much more research is required to optimise them for mass production. PLGA & PLA are promising candidates for scalability are since these materials are widely used across the industry. Operti et al. (2022) demonstrated an encapsulation efficiency of 80% for PLGA nanoparticles encapsulating celecoxib using sonication,24 and in 2024 Maurizii et al. demonstrated 90% encapsulation efficiency for chitosan nanoparticles using microfluidic-assisted ionotropic gelation25.
Fine-tuned function
Interactions between PNPs and biological barriers depends strongly on the physiochemical characteristics of the nanoparticle surface, size, shape and hydrophobicity. One particular associated challenges is the electrostatic and hydrophobic interactions between PNPs and the mucus barrier and exopolysaccharides (EPS) biofilms present in the pulmonary tracts.26 The PEGylation of PNPs surface gives them a sufficiently hydrophilic surface and improves their ability to penetrate mucus and biofilm layer, improving drug delivery.26 This technique was successfully applied to PEI-g-PEG nanoparticles acting as a nanocarrier of mRNA for gene transfection specifically targeting lung tissue when observed in mice models.27 Notably, these functionalised PNPs could be stored in lyophilised form at -20°C for over 4 months without a measurable change in particle size or transfection activity, demonstrating exceptional stability.27 Additionally, recent studies have even indicated that replacing PEG functionalization of nanoparticles with zwitterionic polymers may also improve nebuliser stability, reduce aggregation and delivery efficacy.28
PNPs can also be functionalised to be able to exist more stably in the microenvironment of the lung. Reactive oxygen species (ROS)-responsive PNPs have emerged as a promising platform for pulmonary drug delivery, offering targeted and efficient therapeutic modulation of lung injury microenvironments. When lung tissue is damaged, cells release large amounts of ROS species into the lung microenvironment, the excessive accumulation of which can lead to oxidative damage and increase inflammation and tissue injury. In a recent study by Muhammad et al. (2022), this ROS response was leveraged to hallmark of lung injury and trigger therapeutic release from PNPs.29 By incorporating ROS-sensitive linkages within the polymer matrix, PNPs loaded with anti-inflammatory dexamethasone can achieve more targeted drug release, improving local anti-inflammatory responses, minimizing systemic exposure and maximizing therapeutic efficacy. Compared to free dexamethasone, the PNPs exhibit superior pharmacokinetics and biodistribution, maximising drug retention in pulmonary tissues while minimising off-target effects. This study highlights the promise of ROS-responsive PNPs as a game changer for treating lung diseases driven by oxidative stress, like ALI and COPD, opening the door for future breakthroughs in pulmonary nanomedicine.
In pulmonary diseases like chronic obstructive pulmonary disease (COPD), pneumonia, and acute lung injury (ALI), thermally triggered PNPs can enhance drug retention in the lungs while reducing off-target toxicity. Additionally, these nanoparticles can be engineered for inhalation delivery, ensuring deep lung penetration and prolonged therapeutic action.
A miniature future
PNPs represent a paradigm shift in pulmonary drug delivery, overcoming physiological barriers through rational design. Their stability, modularity, and compatibility with inhalation devices position them as versatile platforms for treating a huge range of conditions. Future research must address scalability & toxicity challenges and continue to explore combinatorial therapies that leverage the unique physiochemical properties of PNPs. Going forwards, we predict that PNPs will play a central role in the evolution of inhalation technology.
Get in touch if you’d like to find out more.
Written by: Kamaal de Silva, Principal Mechanical Engineer and Ethan Miller, Senior Biophysicist
References:
- Translate Bio, Inc. A Phase 1/2, Randomized, Double-Blinded, Placebo-Controlled, Combined Single and Multiple Ascending Dose Study Evaluating the Safety, Tolerability, and Biological Activity of MRT5005 Administered by Nebulization to Adult Subjects With Cystic Fibrosis. clinicaltrials.gov; 2020. Accessed March 14, 2025. https://clinicaltrials.gov/study/NCT03375047
- Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release. 2012;161(2):505-522. doi:10.1016/j.jconrel.2012.01.043
- Maloney Norcross SE, Levin LPK, Hickey AJ, Hill DB. Biopolymeric Inhalable Dry Powders for Pulmonary Drug Delivery. Pharmaceuticals. 2024;17(12):1628. doi:10.3390/ph17121628
- Chan HW, Chow S, Zhang X, Zhao Y, Tong HHY, Chow SF. Inhalable Nanoparticle-based Dry Powder Formulations for Respiratory Diseases: Challenges and Strategies for Translational Research. AAPS PharmSciTech. 2023;24(4):98. doi:10.1208/s12249-023-02559-y
- Rudokas M, Najlah M, Alhnan MA, Elhissi A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med Princ Pract. 2016;25(Suppl 2):60-72. doi:10.1159/000445116
- Baloira A, Abad A, Fuster A, et al. Lung Deposition and Inspiratory Flow Rate in Patients with Chronic Obstructive Pulmonary Disease Using Different Inhalation Devices: A Systematic Literature Review and Expert Opinion. Int J Chron Obstruct Pulmon Dis. 2021;16:1021-1033. doi:10.2147/COPD.S297980
- ZEPHEX®-134a-MEDICAL-PROPELLANT-BROCHURE-1.pdf. Accessed March 13, 2025. https://www.kouraglobal.com/wp-content/uploads/ZEPHEX%C2%AE-134a-MEDICAL-PROPELLANT-BROCHURE-1.pdf
- Greene SF, Nikula KJ, Poulin D, McInally K, Reynolds JA. Long-Term Nonclinical Pulmonary Safety Assessment of Afrezza, a Novel Insulin Inhalation Powder. Toxicol Pathol. 2021;49(2):334-348. doi:10.1177/0192623320960420
- Miao H, Huang K, Li Y, et al. Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA. International Journal of Pharmaceutics. 2023;640:123050. doi:10.1016/j.ijpharm.2023.123050
- Noymer P, Myers D, Glazer M, Fishman R, Cassella J. The Staccato ® System : Inhaler Design Characteristics for Rapid Treatment of CNS Disorders. In: ; 2010. Accessed March 12, 2025. https://www.semanticscholar.org/paper/The-Staccato-%C2%AE-System-%3A-Inhaler-Design-for-Rapid-of-Noymer-Myers/6ad43fd47f34a5377d2b069c76846db4b1b137a3
- Adorni G, Seifert G, Buttini F, et al. Aerosolization Performance of Jet Nebulizers and Biopharmaceutical Aspects. Pharmaceutics. 2019;11(8):406. doi:10.3390/pharmaceutics11080406
- Elhissi AMA, Taylor KMG. Delivery of liposomes generated from proliposomes using air-jet, ultrasonic, and vibrating-mesh nebulisers. Journal of Drug Delivery Science and Technology. 2005;15(4):261-265. doi:10.1016/S1773-2247(05)50047-9
- Katial RK, Reisner C, Buchmeier A, Bartelson BB, Nelson HS. Comparison of three commercial ultrasonic nebulizers. Annals of Allergy, Asthma & Immunology. 2000;84(2):255-261. doi:10.1016/S1081-1206(10)62763-9
- Ari A, Fink JB. Delivered dose with jet and mesh nebulisers during spontaneous breathing, noninvasive ventilation and mechanical ventilation using adult lung models. ERJ Open Res. 2021;7(3):00027-02021. doi:10.1183/23120541.00027-2021
- Waldrep JC, Berlinski A, Dhand R. Comparative Analysis of Methods to Measure Aerosols Generated by a Vibrating Mesh Nebulizer. Journal of Aerosol Medicine. 2007;20(3):310-319. doi:10.1089/jam.2007.0538
- Mendes BB, Conniot J, Avital A, et al. Nanodelivery of nucleic acids. Nat Rev Methods Primers. 2022;2(1):1-21. doi:10.1038/s43586-022-00104-y
- Schuster BS, Suk JS, Woodworth GF, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34(13):3439-3446. doi:10.1016/j.biomaterials.2013.01.064
- Champion JA, Walker A, Mitragotri S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm Res. 2008;25(8):1815-1821. doi:10.1007/s11095-008-9562-y
- Zielińska A, Carreiró F, Oliveira AM, et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020;25(16):3731. doi:10.3390/molecules25163731
- Crucho CIC, Barros MT. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Materials Science and Engineering: C. 2017;80:771-784. doi:10.1016/j.msec.2017.06.004
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101-124. doi:10.1038/s41573-020-0090-8
- Cojocaru E, Petriș OR, Cojocaru C. Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine. Pharmaceuticals (Basel). 2024;17(8):1059. doi:10.3390/ph17081059
- Subraveti SN, Wilson BK, Bizmark N, Liu J, Prud’homme RK. Synthesizing Lipid Nanoparticles by Turbulent Flow in Confined Impinging Jet Mixers. J Vis Exp. 2024;(210). doi:10.3791/67047
- Operti MC, Bernhardt A, Sincari V, et al. Industrial Scale Manufacturing and Downstream Processing of PLGA-Based Nanomedicines Suitable for Fully Continuous Operation. Pharmaceutics. 2022;14(2):276. doi:10.3390/pharmaceutics14020276
- Maurizii G, Moroni S, Jimènez Núnez JV, et al. Non-invasive peptides delivery using chitosan nanoparticles assembled via scalable microfluidic technology. Carbohydrate Polymer Technologies and Applications. 2024;7:100424. doi:10.1016/j.carpta.2024.100424
- Huang Z, Kłodzińska SN, Wan F, Nielsen HM. Nanoparticle-mediated pulmonary drug delivery: state of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Delivery and Translational Research. 2021;11(4):1634-1654. doi:10.1007/s13346-021-00954-1
- Ke X, Shelton L, Hu Y, et al. Surface-Functionalized PEGylated Nanoparticles Deliver Messenger RNA to Pulmonary Immune Cells. ACS Applied Materials and Interfaces. 2020;12(32):35835-35844. doi:10.1021/acsami.0c08268
- Jiang AY, Lathwal S, Meng S, et al. Zwitterionic Polymer-Functionalized Lipid Nanoparticles for the Nebulized Delivery of mRNA. Journal of the American Chemical Society. Published online November 2024. doi:10.1021/jacs.4c11347
- Muhammad W, Zhu J, Zhai Z, et al. ROS-responsive polymer nanoparticles with enhanced loading of dexamethasone effectively modulate the lung injury microenvironment. Acta Biomaterialia. 2022;148:258-270. doi:10.1016/j.actbio.2022.06.024