Taking the Sting out of Vaccines
28 November 2022
Cast your mind into the future and imagine the world in the grips of the next global respiratory pandemic. We have the monumental task of vaccinating the world against a novel pathogen. How should we achieve this?
Our current default is long lines of people stood two meters apart queuing for an injection at a clinic, masked to reduce transmission of an air-borne disease, and hundreds of clinical staff and volunteers trained up to administer the vaccines. Furthermore, the injectable vaccines often need cold-chain transportation of precious vaccines to remote communities across the world.
Alternatively, what if we could post an inhaler directly to people’s homes? Over the past few years there has been growing interest in inhaled and intranasal delivery of vaccines. Inhalation has several advantages over the traditional needle-based method, particularly when considering tackling the next respiratory epidemic or pandemic. This article will examine some of the key advantages and remaining challenges and summarise some of the recent progress that has been made.
Key advantages
1. No needle
The removal of the needle improves patient comfort and safety. Needles present the risk of needle-stick injuries and needle re-use, increasing the chance of cross contamination. A trained healthcare professional is required to administer the vaccine, creating a potential bottleneck for mass vaccination campaigns, particularly in non-industri
alized countries and remote areas (Figure 1). Additionally, removing the needle may reduce vaccine hesitancy and increase compliance, as up to 10% of the UK population suffer from needle-phobia1 , and the rate in other countries may be similar.
Inhaled or intranasal delivery of vaccines could remove the need for a trained healthcare professional to be present in the majority of instances. The device could be collected from designated collection points, such as pharmacies or local grocery stores, or even delivered directly to people homes, to be self-administered at home. However, there are hurdles to achieving this idealised situation, as discussed later in this article.
Inhaled vaccines could yield sustainability improvements too. The quantity of unrecyclable, contaminated sharps generated by COVID vaccine syringes in the UK alone would fill around 10 double-decker buses [1]. If a dry powder inhaler made from a few simple plastic parts could do the same job, it could easily be recycled into high-grade feedstock. Furthermore, dispensing with the cold chain – as discussed later – could have saved about 100 GJ of energy, equivalent to running your kettle every day and every night non-stop for 2 years[2].
2. Mucosal immunity
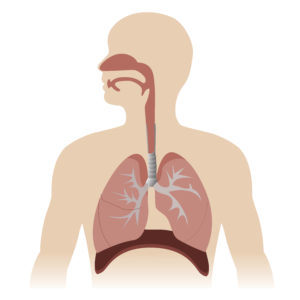
Vaccines delivered via injection generally induce a systematic immune response, that is not specifically directed at the pathogen’s region of infection, such as mucosal sites2. In contrast, nasal vaccines are able to instigate mucosal immunity directly at the site of infection3 (Figure 2), which may provide a higher degree of protection against respiratory pathogens.
The systemic immune response induced by intramuscular vaccines provides protection to the lower respiratory tract and prevents severe complications following a respiratory infection. However, initial infection and early disease symptoms may not be prevented because the upper respiratory tract is not protected4. The pathogens have the opportunity to invade cells, multiply and spread before they can be identified and neutralised by a systemic immune response.
In contrast, mucosal vaccines are able to induce mucosal immunity as well as systemic immunity against a pathogen. A specialised response of mucosal associated lymphoid tissue instigated by a vaccine, enables pathogens to be neutralised before they can cause an infection2. This prevents early disease symptoms and can reduce transmissibility of the pathogen. As we have all become aware of during the COVID-19 pandemic, reducing transmissibility is essential in slowing down the spread of a
new pathogen or stain. Moreover, this additional immune response is not limited to the site of the vaccination, it also offers augmented protection in other adjacent or related mucosal tissues5.An intra-nasal vaccine may therefore also induce mucosal immunity in lung tissue.
1. Removing the cold-chain
Another exciting advantage of inhaled and intra-nasal vaccines is the option to deliver the vaccine as a dry-powder, which would remove the need for cold-chain transportation and storage. Most vaccines need to be stored between 2oC and 8oC to maintain their potency. The COVID-19 pandemic accelerated the roll-out of novel vaccine platforms, such as mRNA-Based vaccines. These allowed for rapid development and roll-out, but with the disadvantage of extremely cold shelf-life temperature requirements. The BioNTech/Pfizer COVID-19 vaccine, for example, requires storage at −80 °C with a shelf life up to 6 months6 . Although most can be transferred to fridge temperatures for up to 30 days, the extreme cold-chain requirements can make the vaccines inaccessible for large parts of the world and can lead to vaccines being discarded, due to expiring the time period at which they can be stored above super-cooled temperatures. Hence, there is interest in improving the stability of mRNA-based vaccines6 as well as that of traditional vaccines, including formulation as a dry powder. Formulating complex biologics into dry-powders has its own challenges, as discussed later in this article, but thin-film freeze-drying is showing promising results.
The benefits of reducing the dependence on cold-chain logistics for facilitating mass-vaccination campaigns across the globe cannot be underestimated: increasing access, reducing vaccine wastage, and reducing the financial and environmental burden.
Recent developments
To date, the only widely used vaccine targeted at the respiratory system is an intranasal influenza vaccine known as FluMist in the US and as Fluenz Tetra in the UK. With the COVID pandemic, there has been increased interest in intranasal and inhaled vaccines. According to Nature, around 100 nasal or oral vaccines are currently under investigation7. Several of these have received limited approval for human use, including the CanSino Biologics vaccine in China, and the Razi Vaccine and Serum Research Institute vaccine in Iran. Phase III trials for a number of others are under way. However, the development of the AstraZeneca vaccine as a nasal spray has hit a road-block with weak results in Phase I trials8.
The vaccines currently under research belong to a variety of different types, including viral vector, protein sub-unit and live virus, though they all appear to revolve around delivery in a spray or drops rather than a dry powder, which does not come with the benefits of removing the cold chain.
Some dry powder inhalers already on the market, such as TwinCaps (Hovione) 9, may be suitable for the delivery of a dry powder vaccine. However, active research into dry powder formulations of vaccines is less advanced than liquid formulations, though a 2019 study reported delivery of a dry powder influenza vaccine in ferrets10.
Despite the recent disappointing result with the AstraZeneca vaccine, the outlook for respiratory mucosal vaccination and the untapped potential of dry powder formulations is promising for the coming years.
Challenges
This article has highlighted some of the key advantages of inhaled and intranasal delivery of vaccines. However, challenges remain to the successful roll-out of an effective respiratory vaccine, such as:
Formulation
The respiratory system has many defence mechanisms, such as mucociliary clearance, in place to protect us from foreign micro-organisms and particles, including the constituents of vaccines. A safe, effective vaccine must bypass these mechanisms to induce the appropriate immune response, without causing severe adverse effects. In order to achieve this, selection of vaccine platform and adjuvants, if required, must be taken into careful consideration.
Dry powders have stability and aerodynamic advantages but formulating complex biologics into dry powders is no easy task. Physical and chemical degradation can occur during the drying process and so the choice of technique must be carefully considered. Traditional methods include spray-drying and freeze-drying (lypophization). However, both have disadvantages for inhaled biologics: spray-drying uses heat which can denature proteins, and the mechanical stresses involves in freeze-drying methods can cause protein aggregation11. Thin-film freeze-drying is an exciting new addition to the field12, which has been shown to be a viable method for converting vaccines into dry-powders13,14, and is likely to become a big player.
Usability and training
Whilst inhaled vaccines may not need a trained medical personal to be present for safety reasons, the idealised situation of self-administration at home without supervision is problematic. How do we prove that the user took the vaccine, in order to authorise a “vaccine passport” or equivalent? Furthermore, how do we prove they took it correctly? Dose accuracy is inherently dependant on correct inhalation and usage by the patient for inhaled and intranasal medicines. DPIs, for example, require a sharp intake of breath, which regular users will receive training for. A mass vaccination campaign using a dry-powder inhaler would require educating the masses effectively on the correct usage, otherwise the efficacy would be severely reduced.
Dose accuracy
The dose accuracy will be more variable than for an injected vaccine, even if administered correctly. In addition to incorrect usage, the delivered dose could also vary due to factors such as:
- Inspiratory flow rate
- Mucosal state, for example having a cold
- Comorbidities such as asthma or COPD
- Nasal or lung geometry
- Ambient humidity and the time taken from removing packaging to taking the drug, both of which affect dry powders rapidly due to changes in their adhesion and cohesion properties
Furthermore, it is more difficult to measure dose accuracy for an inhaled, locally acting drug.
Final remarks
Inhaled and intranasal vaccines have some key advantages over traditional needle-based methods, as highlighted in the article. But there are challenges to overcome, if they are to be relied on for future mass vaccination campaigns.
Contact us today to find out more.
Written by Heather Jameson, Omar Shah and Juliette Ngan
As seen in OndrugDelivery
References:
- Injection Phobia – Anxiety UK. https://www.anxietyuk.org.uk/anxiety-type/injection-phobia/.
- Heida, R., Hinrichs, W. L. J. & Frijlink, H. W. Inhaled vaccine delivery in the combat against respiratory viruses: a 2021 overview of recent developments and implications for COVID-19. https://doi.org/10.1080/14760584.2021.1903878 21, 957–974 (2021).
- An, X. et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience 24, (2021).
- Amorij, J., Hinrichs, W. L. J., Frijlink, H. W., Wilschut, J. C. & Huckriede, A. Needle-free influenza vaccination. 699–711 (2010) doi:10.1016/S1473-3099(10)70157-2.
- Kurono, Y. The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines. Auris Nasus Larynx 49, 1–10 (2022).
- Uddin, M. N. & Roni, M. A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines (Basel) 9, (2021).
- Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 609, 240–242 (2022).
- Madhavan, M. et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. EBioMedicine 0, 104298 (2022).
- Luczo, J. M. et al. Intranasal powder live attenuated influenza vaccine is thermostable, immunogenic, and protective against homologous challenge in ferrets. npj Vaccines 2021 6:1 6, 1–8 (2021).
- TwinCaps® DPI | Hovione. https://www.hovione.com/products-and-services/contract-manufacturing-services/drug-product/inhalation/devices/twincaps-dpi.
- Williams, R. Improved Formulations to Enable Stable Delivery of Biologics. Biopharm Int 35, 46–49 (2022).
- Hufnagel, S., Sahakijpijarn, S., Moon, C., Cui, Z. & Williams, R. O. The Development of Thin-Film Freezing and Its Application to Improve Delivery of Biologics as Dry Powder Aerosols. KONA Powder and Particle Journal 39, 176–192 (2022).
- Alzhrani, R. F. et al. Thin-film freeze-drying is a viable method to convert vaccines containing aluminum salts from liquid to dry powder. Methods in Molecular Biology 2183, 489–498 (2021).
- Xu, H. et al. Thin-film freeze-drying of a bivalent Norovirus vaccine while maintaining the potency of both antigens. Int J Pharm 609, 121126 (2021).