What are the challenges facing gene therapies?
24 June 2021
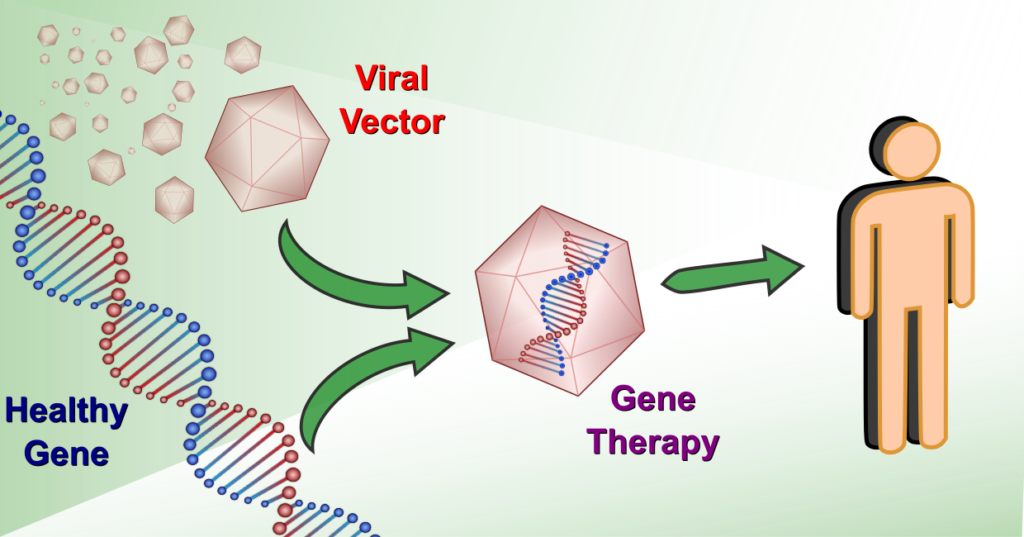
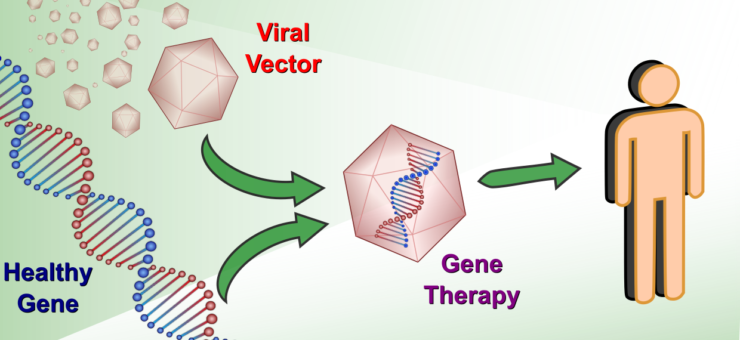
Gene therapies are at the forefront of medical science, looking to treat the rarest of diseases that collectively afflict millions of people worldwide. They typically target genetic disorders, where a genetic defect modifies or prevents the proper functioning of cells, often leading to life-threatening conditions. Where current treatments only manage symptoms and can be burdensome or non-existent, gene therapies aim to deliver a curative treatment in a single injection. The basic principle is to introduce new functional genetic material into the cells, most commonly via a non-pathogenic virus carrier (viral vector), that either replaces or counteracts the defective genetic code. The first gene therapy was approved over two decades ago [1], but since then only around 15 more have been approved worldwide. While momentum is building in the field, and the approval rate has increased, the path to a fully developed gene therapy still has significant challenges.
Gene therapies have the potential power to alter millions of people’s lives, but this potential is spread across thousands of different genetic diseases. As a result, a single disease may only effect 1 in 100,000 people, and this rarity is an underlying factor to many of the challenges faced by gene therapies. When few occurrences of a disease exist developing thorough knowledge of its biological nature is a difficult and time-consuming process, necessitating strong collaborations between academia and industry. A successful therapy requires a detailed understanding of the genetic defect, its biological impact, and the specific cells that require targeting. Even then, an appropriate and efficient vector for delivering the new genetic material to the specific cells is also needed. While building the knowledge and developing these therapies is challenging, ensuring their safety and efficacy is no easier. Rare diseases inherently have few people able to participate in clinical trials and possible pre-existing antibodies to viral vectors can restrict this number further. These small enrolment numbers necessitate careful trial design to ensure confidence in the treatment’s efficacy [3]. Moreover, difficulty in recruitment, among other factors, can double the length of a rare disease clinical trial compared to standard trials [2], while specific guidelines to ensure long term safety of gene therapies can also lengthen the time for validation [4].
Additionally, there is the high cost and low efficiency of the manufacturing processes for viral vectors and integration of the new genetic material [5]. The development of vectors is often started in a university setting, where small batch methods are adequate but not viable options for large scale manufacturing. These semi-manual adherent-cell methods require substrates for the process—not suitable large volume production—and expensive high grade input materials that can be difficult to obtain.
Finally, the compounding of long development times, efficiency in manufacture, and limited number of potential recipients leads to the headline-grabbing price tags associated with these treatments—the recent approval of Zolgensma (Novartis Gene Therapies) for use by the NHS costs £1,790,000 per treatment [6]. Within a single payer system these types of costs can be justified when compared to cumulative costs of symptom management treatments. However, healthcare systems built around multiple private insurance companies, where individuals may move between providers, are not designed to cope with such large costs for a single treatment and could present significant barriers to uptake.
In the face of these challenges great progress is still being made and the field of gene therapy is viewed as close to a tipping point. Continued research interest, new trial formats [7], investment in and development of scaled-up manufacture [8], and patient outcome related pricing [9] all indicate much needed progress. The US FDA expects that the number of therapies meeting approval will increase rapidly, up to around 10 to 20 per year by 2025 [10].
Many gene therapies need to be injected or infused into the body. Given that these are the most expensive treatments ever created, they need delivery devices with the highest levels of accuracy, efficiency, and reliability. However, sometimes gene therapies also place non-standard requirements on the device, such as different needles to access different parts of the body, or flowrate control, or pressure control and so on. At Springboard we have a wealth of expertise in medical delivery devices and if you are working at a company developing gene therapies, do not hesitate to get in touch.
[1] Shahryari A, Saghaeian Jazi M, Mohammadi S, et al. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front Genet. (2019) 10:868. doi:10.3389/fgene.2019.00868
[1] Hilgers R. Design and analysis of clinical trials for small rare disease populations. J Rare Dis Res Treat. (2016) 1:53. doi:10.29245/2572-9411/2016/3.1054
[2] Jayasundara K, Hollis A, Krahn M, Mamdani M, Hoch JS, Grootendorst P. Estimating the clinical cost of drug development for orphan versus non-orphan drugs. Orphanet J Rare Dis. (2019) 14:12. doi:10.1186/s13023-018-0990-4
[3] Gene therapy needs a long-term approach. Nat Med. (2021) 27:563. doi:10.1038/s41591-021-01333-6
[4] Srivastava A, Mallela KMG, Deorkar N, Brophy G. Manufacturing Challenges and Rational Formulation Development for AAV viral vectors. J Pharm Sci. (2021) 000:1. doi:10.1016/j.xphs.2021.03.024
[6] Day S, Jonker AH, Lau LPL, et al. Recommendations for the design of small population clinical trials. Orphanet J Rare Dis. (2018) 13:195. doi:10.1186/s13023-018-0931-2
[7] Pfizer, Novartis lead $2 billion spending spree on gene therapy production | Reuters
[8] Jørgensen J, Hanna E, Kefalas P. Outcomes-based reimbursement for gene therapies in practice: the experience of recently launched CAR-T cell therapies in major European countries. J Mark Access Heal Policy. (2020) 8:1715536. doi:10.1080/20016689.2020.1715536
[9] Scott Gottlieb, “Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies,” Food and Drug Administration, press release, January 15, 2019, fda.gov.
— Joe Batley