Why are medical device product developments late? – Part one
11 January 2023
In part one of a two part series we take a look at why medical device developments are often delayed, with some common causes that we have seen over the years. In part two, we look at some solutions to ensure that development plans stay on schedule.
Companies both large and small consistently seem to underestimate how long it will take to develop a medical device. Springboard are experienced at medical device design and we frequently hear from other companies that their product was due on the market last year but due to delays in their development the product has not been yet released. Here are common reasons why medical device product developments can overrun:
- Poorly formulated inputs. Medical devices are designed to meet requirements which have been formulated to address well understood user needs. This is what informs design decisions. Any changes to requirements mid-development can invalidate these decisions and result in dramatically increased development effort to rectify.
- Loosely defined technical requirements. A medical device must be verified against the specified performance. If the specified technical performance is not well defined then this will make verification tests hard to define and introduce a risk of producing a device which will fail validation tests.
- Requirements driven by technical ambition rather than user need. The technical team will understandably want to produce a product with the best performance possible. Therefore, there is normally a tendency to push requirements to the limit of what is achievable technically. This doesn’t always mean a more desirable product as the user doesn’t need that performance, but it does mean an increase in development time and risk of verification failures. A well defined product development will differentiate very strictly between “must have” and “nice to have” requirements.
- Underestimation of the time required for quality. Medical devices are highly regulated and must be developed following a process. Most companies will have a quality team which ensure this process is followed and the development plan must allow time for document review, iteration and control.
- System integration. Modern medical devices are complex with multiple disciplines involved in the development. When the final prototype is put together and all the systems are combined in one device then this can uncover problems which were not evident when the individual systems were tested in isolation.
- A plan for the best case. There is a tendency to have a plan which assumes everything will go well. This is understandable as there will be pressure for the product to be ready and it is hard to plan for problems which are not known at the start. But even if the plan represents the average time for the development, then there is a 50% chance of being late. Furthermore, many deviations from plans produce stepwise delays – whilst the late delivery of a key component might push a timeline back by one week an unexpected test failure could require a whole new design integration (see figure).
- Insufficient review. The later design issues are caught the more problematic and time consuming they are to fix. Many problems would be discovered early with thorough, independent project reviews.
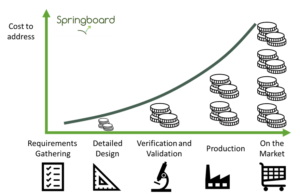
Escalating costs associated with delays
These are some of the most common issues which we have come across when developing medical devices. Do they sound familiar to anyone? Look out for part 2 where we will discuss methods to avoid these issues.
Written by Liam Malone, Principal Physicist and Management Team Member


