Wearable injectors news and trends
26 September 2022
The on-body delivery system (OBDS) market is changing rapidly, and it has become clear that OBDS devices are critical to the delivery of some new drugs in development. The OBDS market is relatively young, which lends itself to exciting innovations and high potential growth rate. This article reviews recent milestones, summarises the current state of play, and proposes directions for the future.
Before launching into the details, we need to clarify which OBDSs we are discussing. By far the largest market for wearable injectors is diabetes. However, ambulatory insulin infusion pumps will be considered out of scope of this article because they deserve an article all to themselves. They are focused on one therapy where the flowrate is clinically relevant, and their requirements are covered by IEC-60601-2-24 (if electronic) or ISO 28620 (if non-electronic).
For this article we shall focus on OBDSs where the flowrate is not clinically relevant (other than avoiding the flowrate being too high which could cause discomfort or leaks), and their requirements are covered by ISO 11608-6:2022. Such OBDSs can be used for a wide and ever-increasing range of indications and drugs, particularly where the relatively high volume, viscosity, or dose timing makes autoinjectors inappropriate. Examples are pegfilgrastim to reduce infections after chemotherapy, evolocumab for reducing cholesterol, drugs for Parkinson’s Disease, and more.
Recent milestones
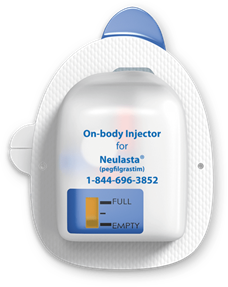
The first launch of the modern generation of non-insulin OBDSs was Insulet’s Onpro® for Amgen’s Neulasta® drug in 2015.[1] This is a modified version of the Omnipod insulin pump and delivers the injection in one bolus approximately 27 hours after the device is filled from a prefilled syringe by a healthcare professional and attached to the patient’s body. Since its launch, over 1 million patients have used the Onpro OBDS to deliver Neulasta.[2] The drive mechanism uses shape memory alloy (“muscle wire”) to reciprocate a lever arm which rotates a ratchet wheel and leadscrew to push the plunger. The drug container is a custom oval plastic cartridge.
West’s SmartDose 3.5 mL was launched in 2016 for Amgen’s Repatha® and branded “Pushtronex®”.[3] In 2019, scPharmaceuticals (MA, US) announced its intent to go to market with West’s SmartDose® 10 injector for FUROSCIX®, a proprietary, subcutaneously delivered furosemide solution for the treatment of worsening heart failure due to congestion[4]. The platform uses an innovative telescopic plunger rod to minimise device size. The primary drug container is a custom cartridge made of cycloolefin polymer.
Enable has supported 3 clinical studies using its enFuse® pump, one of which is published.[5] The enFuse uses a unique approach to drug delivery. The elastomeric technology offers a low, responsive pressure injection that responds to the pressure in the sc tissue and adjusts flow via pressure. Pharmaceutical partners can use their original drug container as the user fills the device just before use, thus inflating an elongated elastomeric drug container. Once on the body and activated, the elastic energy of the drug container expels the drug formulation in “bands” to smooth out the pressure over time. Enable has announced relationships with Lilly, Sanofi, Apellis, Genentech, UCB and CSL Behring[6], and has received USD 215m financing in January 2022.[7]

enFuse® pump vial transfer is 1-click by the patient.
Ypsomed’s YpsoDose single-use injector is an electromechanical pre-filled and pre-assembled patch device for 10 mL glass cartridges. Needle insertion, injection, end of injection feedback and needle safety steps are all performed automatically. The needle remains hidden at all times and made safe after injection and device removal. Ypsomed is scaling up its manufacturing capability for YpsoDose in Switzerland with first clinical studies starting in 2023.

Nemera has completed a formative study on its Symbioze wearable pump and expects to have new prototypes from rapid tooling by the end of 2022. The design has a reusable subassembly containing the motor and electronics, and a disposable subassembly containing the drug container and path. The reusable subassembly checks the identification of the disposable subassembly using NFC before injection. The reusable electronics allows for additional features with minimal impact on cost or the environment, such as Bluetooth connectivity.

BD has announced 2 OBDS platforms that are in development:
- BD Evolve™ On-body Injector is a programmable platform supporting injections up to 3 mL. BD has conducted 9 pre-clinical and 5 human factor studies.
- BD Libertas™ Wearable Injector supports 2 to 5 mL or 5 to 10 mL in its two sizes and is fully mechanical (no electronics or software). The drug container is a nearly standard glass cylinder, available in standard nest and tub configurations. In 2021 BD published a clinical study on the 5 mL variant with 52 subjects to test functionality and acceptability with patients.[7]
Gerresheimer acquired Sensile Medical and now has 3 pumps in its offering[8]:
- SensAir, a pump optimised for delivering large molecules.
- On-body delivery system based on the SenseCore rotating piston pump for small molecules.
- Belt-worn delivery system, currently used by EVER pharma to deliver apomorphine for Parkinson’s disease.

Bespak (now owned by Recipharm) is not actively developing its HFA-powered Lapas wearable injector, and SteadyMed was acquired by United Therapeutics which took the expanding-battery-driven Patch Pump ® out of the running.
Current device strategies
Springboard is in the privileged position of being involved in the development of many of the pumps currently in the pipeline. We always respect confidentiality but can identify some themes in public.
Early in development, the device designer needs to decide whether to use a standard or custom drug container. There is frequent temptation to use a custom drug container to improve device layout, enable novel delivery mechanisms, and improve usability. There are numerous OBDSs in development with custom drug containers (Table 2), and many more that have been discontinued. However, pharmaceutical companies continue to see the drug container as a key risk, especially for prefilled devices, because anything that is not a standard glass pharmaceutical cartridge requires a lot more risk management and evidence before being accepted. The choice of drug container is particularly important for prefilled devices because they typically have strict requirements for drug stability over 2 years shelf life.
Finally, the device designer must choose between two fundamental methods of taking the drug out of the container and delivering it to the patient: either pushing the drug out of the container, or to pulling it out. Each has their advantages and disadvantages. The advantages of one method mirror the disadvantages of the other so we only need to list the advantages (Table 1).
Table 1: Advantages of pushing or pulling the drug out of its container

We can see how different companies are providing pumps with different strategies around drug container and “push” or “pull” (Table 2).
Table 2: Publicly announced pumps categorised by push/pull and drug container

New ISO 11608-6 standard
The ISO 11608 family of international standards has been updated and many were published April 2022.
ISO 11608 part 6 is new and covers on body delivery systems. It only covers fixed-dose delivery systems where the medicinal effect is measured by the dose volume, not the flowrate. In contrast, delivery systems which are designed to deliver given flowrates are covered by IEC 60601-2-24 and ISO 28620. The standard is designed to cover requirements that are not typically present for handheld injection devices, such as:
- Means of attachments to the body.
- Occlusion detection.
- Leakage due to worst-case environments, and orientation.
- Changes in or periods with vibration, temperature, humidity, atmospheric pressure, light exposure, and orientation whilst in use.
- Dose delivery over time.
It also defines test methods for adhesion, dose delivery profiles, and needle/cannula displacement (whilst in the body).
Springboard expects that the additional requirements described in the 11608-6 standard should make it clearer to stakeholders why there can be more development work required to bring an OBDS to market compared to, say, a prefilled syringe. At the same time, the clarity brought by the standard should reduce the time that device developers and regulatory affairs people spend trying to work out the requirements and expectations for new OBDSs.
Challenges
Considerable progress has been made on the main challenges for wearable injectors in recent years:
- Many patients, caregivers and healthcare professionals are familiar with autoinjectors but the use scenarios with bolus injectors are unfamiliar and therefore the occurrence of certain usability risks could be increased. Usability (human factors) is now being considered early and throughout device development according to the main regulatory requirements in the area: FDA guidance “Applying Human Factors and Usability Engineering to Medical Devices”, published February 2016; and IEC 62366-1:2015 “Application of usability engineering to medical devices”. However, at Springboard we are still seeing some companies preferring not to conduct early formative human factors studies which are critical to determining meaningful user requirements specification.
- Parenteral injections need to be sterile. The sterility strategy for prefilled syringes tends to be straightforward (the syringe is sterilised with its needle shield in place then filled in a sterile isolator). However, most OBDSs do not use a prefilled syringe and they need a strategy to both achieve and maintain sterility. This can require some novelty when the drug container is not connected to the needle or cannula before use because there could be a non-sterile region between those two subassemblies. With suitable design, various methods for sterilising the drug path have been used successfully in different devices, such as ethylene oxide, nitrogen dioxide, ultraviolet radiation in situ and others.
Three challenges have continued unabated and may have increased recently:
- Cold chain distribution presents a challenge to device developers. Most biologics need to be transported and stored at refrigerated temperatures. Therefore, if the OBDS is prefilled it must be robust to cold temperature and condensation, which can be challenging for electronics and batteries. In addition, the overall size of the device and its packaging must be minimised because cold chain space is finite, expensive, and has significant environment footprint.
- Demands from patients, payers, and pharmaceutical companies to reduce environmental impact are increasing. We have seen some devices selected on their environmental credentials. Compared to prefilled syringes or autoinjectors, wearable injectors tend to:
- Require more plastics.
- Often need an adhesive patch
- Often need soft cannula insertion and perhaps retraction
- Sometimes need electronics and batteries.
Some wearable injectors employ strategies such as:
- Splitting the device into reusable and disposable subassemblies, and/or
- Increasing the number of reuses by the same patient for a given device, and/or
- Collecting a used device from one patient, reconditioning it, and sending it to another patient to use.
- The Intellectual Property space has become even more crowded and so freedom to operate is a key issue.
Future directions
We can speculate on future directions of development for wearable injectors, and expect the following themes to be:
- Rationalisation of requirements and designs. That is, requirements and designs will simplify as we discover which user experiences are most successful on the market, and in turn which user experiences become expected by patients, carers, healthcare professionals, and payers. For example, over the years various autoinjectors automatically inserted the needle, and there was a button to start the injection, and some autoinjectors retracted the needle automatically after use. The current generation of autoinjectors tend to rely on the patient to insert and retract the needle manually (although it is hidden at all times), and the trigger button has been removed in favour of “push the device onto the body to trigger”. These changes may lead to simpler and smaller devices.
- Tension between adding functionality such as connectivity versus optimising for environmental sustainability.
- Connection with companion diagnostics. This will be the subject of a panel discussion at the Partnerships on Drug Delivery (PODD) conference in Boston in October 2022.
- In time, rationalisation of wearable device suppliers in the market. As a relatively new device category, there is a lot of innovation and competition. Like any other disruptive industry or device category, a handful players may come to dominate the market over the coming years because pharmaceutical customers may prefer proven technologies, tooling and production costs might be amortised for devices already on the market, and some device manufacturers will gain early-mover advantage.
Summary
More than at any other time, in 2022 the world recognises the need for high quality, accessible, and sustainable healthcare. The wearable injector market is in an exciting and pivotal phase with some devices on the market, many more devices racing to clinical studies and some falling by the wayside. Pressures on environmental sustainability, ever increasing usability, and demand for new features mean that the race is far from over.
If you have questions or would like to discuss any points, please do not hesitate to contact us.
Written by Tom Oakley, and featured in OnDrugDelivery
References:
- Amgen Announces Launch Of New Neulasta® (Pegfilgrastim) Delivery Kit”. Press Release, Amgen, Mar 2, 2015.
- “Neulasta® Onpro® helps you fight the risk of infection from home and reduces your risk of being hospitalized”. Patient Brochure, Neulasta®, 2020.
- “West’s SmartDose® Drug Delivery Technology Platform Selected by Amgen for Pushtronex™ System”. Drug Development & Delivery, Jul 2016.
- Patel A, “The Path to Commercialisation for Wearable Drug Delivery Devices”. ONdrugDelivery, Issue 124 (Sep 2021), pp 58–62.
- “Sanofi announces €300 million collaboration with Blackstone Life Sciences to advance an innovative treatment for multiple myeloma”. Press Release, Sanofi, Mar 15, 2022.
- “Enable Injections Announces $215 Million Financing”. Press Release, Enable Injections, Jan 27, 2022.
- Woodley WD et al, “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects”. Clin Transl Sci, 2021, Vol 14(3), pp 859–869.
- https://www.gerresheimer.com/en/drug-delivery-systems/platform-solutions/infusion-systems